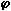 = 0.833)
= 0.833) = 0.833)
= 0.833)
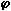 = 0.833 from experiments of sulphur addition from all fuels investigated
= 0.833 from experiments of sulphur addition from all fuels investigated
No large differences were found in the nominal reductions of the NO emissions from the heavy gas oils studied on addition of SO2, which can be observed in Figure 151 (emissions from Orimulsion were reduced further and they will be discussed in subsequent paragraphs). However, the fuels have very different fuel-N and -S contents. No correlation seems to exist between these and the decreases achieved. This suggests that the interaction of S with NO occurs at a stage or by a mechanism which is independent from the amounts of sulphur and nitrogen in the fuel and also of the amounts of NO formed, but somehow dependent on the amount of dopant added. Also, experimental results shown in Figure 148 indicate that the interference of S with NO occurs before 100 mm from the atomiser nozzle, where the concentration of NO was decreased by 9 ppm-wet on addition of SO2. It was postulated in section "5.1.1. Formation of NO at short distances" that NO was formed at short distances predominantly via the fuel-NO mechanism. In addition, Figure 148 shows that the presence of sulphur is not able to modify the concentration of NO in later stages of the flame at  = 0.833.
= 0.833.
Experimental measurements also indicate that a certain proportion of the sulphur dioxide injected as dopant at  = 0.833 is not detected in the early stages of the fuel-air atomisation stage. This is demonstrated by the increase of SO2 concentrations at 100 mm from the atomiser when SO2 dopant is added, which is significantly lower than that at longer distances. This suggests that SO2 is recombined shortly after injection and regenerated at longer distances.
= 0.833 is not detected in the early stages of the fuel-air atomisation stage. This is demonstrated by the increase of SO2 concentrations at 100 mm from the atomiser when SO2 dopant is added, which is significantly lower than that at longer distances. This suggests that SO2 is recombined shortly after injection and regenerated at longer distances.
No one single mechanism so far proposed in the literature reviewed, which is summarised in section "4.3. Interactions between S and N species: Their effect on NOX formation and emission" of chapter I, can justify the experimental results obtained in the drop-tube furnace. It is likely that they are the outcome of various processes operating concurrently.
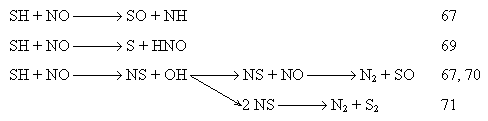
In the early stages of the (fuel - atomisation air) cone, fuel-rich pockets may be formed in which SO2 may be reduced to other sulphurous species. These reduced sulphur species may reduce the concentrations of nitric oxide via reactions such as those proposed by Chagger et al. (Chagger et al. (1991)) :
A peculiar feature of the experimental results obtained at  = 0.833 is an increase of NO2 concentrations concurrent with the decrease of nitric oxide, as shown in Figure 149. However, the final emissions of NO2 in fuel-lean conditions were negligible as it disappears after 200 mm from the atomiser nozzle. The nominal increase of NO2 concentrations is similar to the reduction of the amounts of NO, which may suggest a relationship between both events.
= 0.833 is an increase of NO2 concentrations concurrent with the decrease of nitric oxide, as shown in Figure 149. However, the final emissions of NO2 in fuel-lean conditions were negligible as it disappears after 200 mm from the atomiser nozzle. The nominal increase of NO2 concentrations is similar to the reduction of the amounts of NO, which may suggest a relationship between both events.
NO2 is a nitrogenous compound that has traditionally been overlooked in studies of N-S interactions, even in systems where its formation is likely. A very small number of reports have investigated the interconversion of NO and NO2 aided by sulphur compounds.
Armitage and Cullis (Armitage and Cullis (1971)) proposed a mechanism for the simultaneous decrease of NO and increase of NO2. At low NO concentrations its oxidation proceeds through reaction with SO2 to form NO2 and SO3:
 NO3 reac 74
NO3 reac 74which is a transient stage, followed by
 NO2 + SO3 reac 75
NO2 + SO3 reac 75implying the disappearance of NO and SO2 and the formation of NO2 and SO3. According to Lyon et al. (Lyon et al. (1990)) , the optimum temperature range for these reactions seems to be between 700 and 900 °C. This range of flame temperatures can be encountered in the injection zone of the drop-tube furnace.
Sulphur trioxide could be transformed to SO2 subsequently by means of the reaction
 SO2 + 1/2 O2 reac 51
SO2 + 1/2 O2 reac 51which is known to be displaced to the right at temperatures above 1,000 °C (Cullis et al. (1966)) .
Formation of SO3 could partly explain the reduction of the concentration of oxygen observed on addition of SO2 in fuel-lean conditions. The decrease is more sustained and pronounced for Orimulsion, which also presents the highest concentrations of residual O2 among all fuels investigated at  = 0.833.
= 0.833.
The reduction of NO and the increase of NO2 concentrations in fuel-lean conditions could also be explained in terms of the radical recombination originated by SO2 (Tseregounis and Smith (1984), Zachariah and Smith (1987)) which takes place according to the following reactions (a complete mechanism is shown in section "4.3.1. Thermal-NOX"):
X + SO2 + M  XSO2 + M XSO2 + M
| reac 60 |
X + YSO2  SO2 + XY SO2 + XY
| reac 61 |
where X = O, OH or H and Y = O or H.
As a result, the concentrations of both OH and H radicals will decrease. The formation of NO via the fuel-NO mechanism is thus depleted in the early stages by the lower amounts of OH radicals. Conversely, NO2 formed at low temperatures by reaction of NO with HO2 or RO2 radicals will be increased as the H radicals necessary for its reduction are removed by SO2.
Reductions of NO concentrations in zones of high superequilibrium concentrations were also explained by Tseregounis and Smith (Tseregounis and Smith (1983)) in terms of radical recombination, which can alter the amine reaction subsystem:
 NHi-1 + HX reac 98
NHi-1 + HX reac 98where X: O, H, OH and i: 1, 2, 3.
by removing X radicals. This would shift the balance of this reaction to the left. NO formation would be impeded as it is formed from N (less N radicals would be formed), and NO recombination would be promoted as it forms N2 with NHi via reaction 87 (more NHi radicals would be available).
Chen et al.. (Chen et al. (1984)) found large decreases of OH radicals when SO2 was added to a propane flame. The decrease of OH concentrations was most accentuated under fuel-lean conditions. Durie et al. (Durie et al. (1971)) reviewed the feasibility of the recombination of OH radicals by SO2 by reactions:
OH + SO2 + M  HOSO2 + M HOSO2 + M
| reac 99 |
HOSO2 + OH  H2O2 + SO2 H2O2 + SO2
| reac 100 |
but deemed it to be unlikely in C3H8 fuel-rich flames as the product, H2O2, is unstable. However, enhanced H2O2 concentrations in the drop tube furnace could lead to higher HO2 concentrations (for instance, by reaction of H2O2 with oxygen or OH radicals (Zamansky et al. (1996)) ). This could, in turn, promote the formation of NO2 in the early stages of the combustion process, where the temperature is still low.
In addition to the heavy gas oils, Orimulsion was studied under fuel-lean conditions. The reduction of NO emissions from this fuel was much larger than that from the heavy gas oils. This suggests that the mechanism for NO removal is greatly affected by the presence of water from the fuel or by the inherent characteristics of this heavy fuel. However, the addition of sulphur did not cause, in this case, an increase of the emissions of NO2.
Although in small amounts, it has been suggested that the introduction of water in a combustion system leads to the enhanced production of OH radicals by means of (Dryer (1977)) :
H2O + H  H2 + OH H2 + OH
| reac -7 |
H2O + O  OH + OH OH + OH
| reac 34 |
This would lead to lower concentrations of H and O radicals, as the higher amounts of OH radicals displace the radical pool in such a direction. Higher concentrations of OH could be counteracted partly by recombination by SO2. However, this would lead to increased concentrations of NO2, which did not occur. If H and O radicals are more effective than OH in the formation of NHi and NO, respectively, their recombination could cause a larger proportional effect on those species.
Decreases of OH radical concentrations have been observed by Tang et al. (Tang et al. (1981)) in fuel-lean hexane flames doped with pyridine and S as tertiary butyl mercaptan. Reduced OH concentrations accounted for reductions in the concentrations of nitric oxide.
N2O emissions were also measured at  = 0.833. Orimulsion generated higher emissions of nitrous oxide than the heavy oils, but addition of SO2 did not have a marked effect on them from any of the fuels studied.
= 0.833. Orimulsion generated higher emissions of nitrous oxide than the heavy oils, but addition of SO2 did not have a marked effect on them from any of the fuels studied.
 Previous |  Table of Contents |  Next |