 = 1.000)
= 1.000) = 1.000)
= 1.000)Increases in the measured concentrations of SO2 when increasing amounts of dopant were added were lower than those calculated from the flow of SO2 dopant, as sulphur dioxide was reduced to other species in this oxygen-deficient conditions.
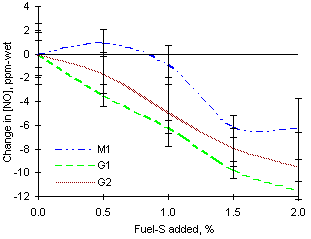
 = 1.000 from experiments of sulphur addition from all fuels investigated
= 1.000 from experiments of sulphur addition from all fuels investigatedExperiments showed no change of NO concentrations at 100 mm (see Figure 148). The interaction of sulphur with NOX species occurs later in the gas path, with concentrations of NO decreasing gradually after 100 mm, and increases of those of NO2. The extent of both interactions was different as NO2 was increased to a larger extent than NO was reduced. This may show that the mechanisms for NO formation and NO2 disappearance apply separately. Figure 139 shows the change of the [NO]/[NO2] ratio with increasing amounts of SO2 under stoichiometric conditions. Whilst NO2 is reduced by reaction with H radicals, NO is formed in reactions with O and OH radicals. As the amounts of SO2 increase NO will be prevented from being formed to a larger extent whereas NO2 will be not reduced. As a result, the ratio [NO]/[NO2] will decrease.
Figures 148 and 149 show that the reduction of NO and the increase of NO2 concentrations occur gradually along the flame path. As the formation of fuel-NO is delayed in lower concentrations of oxygen than those at  = 0.833, the radical recombination by SO2 acts by withdrawing H and OH radicals.
= 0.833, the radical recombination by SO2 acts by withdrawing H and OH radicals.
Tseregounis and Smith (Tseregounis and Smith (1983)) point out that NO can be reduced to N2 by the action of amine species. Withdrawal of H radicals by means of SO2 addition would result in lower amounts of N radicals and higher amounts of NHi radicals.
In a similar way the increase of NO2 concentrations can be explained by the radical recombination assisted by SO2. NO2 is known to disappear by reaction with radicals such as O and H, although the amount of O radicals is expected to be low in these conditions. Thus, further reduced concentrations of these radicals will lead to higher concentrations of NO2.
 Previous |  Table of Contents |  Next |