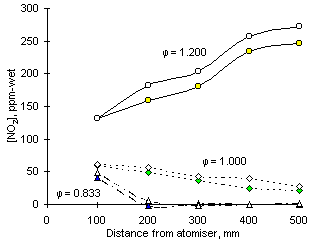
Figure 149: Experimental readings of NO2 from fuel M1 at three equivalence ratios, S addition and selected sampling distances. Solid symbols: air; open symbols: addition of 2.0 % S
Whenever formed, the effect of SO2 addition was to increase the concentrations of NO2. This can be seen in Figure 149.

At 0.833 equivalence ratio (fuel-lean) small amounts of NO2 were formed at short distances from the atomiser nozzle (41 ppm-wet). However, it disappeared rapidly within the ensuing 100 mm. SO2 increased NO2 concentrations by 8 ppm-wet, but after 200 mm the amounts of NO2 remaining were negligible and thus sulphur could no longer exercise any effect.
In stoichiometric conditions the formation and emission of NO2 is significant. At the closest distance investigated (100 mm) 60 ppm-wet NO2 were measured, but no S-N interaction was observed as sulphur was not able to modify that amount. The concentration of NO2 decreased as the sampling probe was displaced away from the atomising nozzle, but the interaction with sulphur raised as sulphur addition increased the concentration of nitrogen dioxide at all other sampling locations. Eventually, emissions of NO2 were raised by 7 ppm-wet, which represents 33 % of the emissions without SO2 addition.
The effect of sulphur is most intense at fuel-rich equivalence ratio. At 100 mm there is no difference in the amounts of NO2 found with or without addition of SO2. However, within the following 100 mm an increment of 23 ppm-wet was established by the addition of SO2. More nitrogen dioxide is formed subsequently, but the increase of NO2 concentration is sustained steadily up to the point of exhaust. NO2 concentrations were increased by 26 ppm-wet from 247 ppm-wet on exhaust at  = 1.200.
= 1.200.
 Previous |  Table of Contents |  Next |