 = 1.000. Addition of sulphur had almost no effect on NO emissions at
= 1.000. Addition of sulphur had almost no effect on NO emissions at  = 1.200.
= 1.200.
The addition of sulphur caused decreases in the exhaust concentrations of NO in fuel-lean and stoichiometric conditions only. However, unlike in previous experiments of SO2 addition, in this case the largest reduction of NO emissions was achieved at  = 1.000. Addition of sulphur had almost no effect on NO emissions at
= 1.000. Addition of sulphur had almost no effect on NO emissions at  = 1.200.
= 1.200.
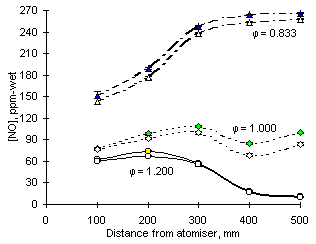
In fuel-lean conditions the decrease of NO concentrations caused by SO2 addition starts at an early stage of the combustion process. Gas samples withdrawn at 100 mm from the atomiser nozzle already reveal a reduction of 9 ppm-wet with the injection of 2 % sulphur on an initial concentration of 152 ppm-wet. Although the concentration of nitric oxide increases subsequently up to a maximum of 266 ppm-wet, the reduction of NO caused by SO2 addition remains almost invariable throughout the combustion process.
In stoichiometric conditions the decrease of NO concentrations occurs progressively. Sampling at 100 mm reveals no change with addition of SO2. As combustion progresses downstream the concentration of NO rises to a maximum value at 300 mm, followed by a slight decrease and a new increase. Along the gas path SO2 is able to reduce the concentration of NO by increasing amounts; eventually a decrease of 16 ppm-wet from 100 ppm-wet is achieved at 500 mm from the atomiser nozzle.
Fuel-rich conditions provide the environment for the lowest formation of nitric oxide. In these conditions the concentration of NO is maximum at 200 mm from the atomiser (67 ppm-wet), followed by a decline and the final emission of 11 ppm-wet. SO2 modifies the concentration of NO only at the point of maximum concentration, and then only with a reduction of 7 ppm-wet. Experimental results at other sampling locations revealed no effect of sulphur on NO formation.
 Previous |  Table of Contents |  Next |