 = 1.200)
= 1.200) = 1.200)
= 1.200)Another effect observed under fuel-rich conditions was a lower increase of the measured concentrations of sulphur dioxide than those calculated. This is due to the transformation of SO2 to other reduced sulphur species in this oxygen-deficient environment (see Figure 99 for a graph detailing the distribution of sulphur species according to the equivalence ratio).
The emissions of NO were low from all fuels studied. However, a better understanding of the influence of sulphur on nitric oxide is given by the sampling at various distances from the atomiser, as shown in Figure 148. SO2 affected NO only at and around 200 mm distance, where it is still undergoing formation or is reaching its maximum concentration. The concentration of nitric oxide was reduced by 7 ppm-wet at 200 mm distance. For the next 100 mm SO2 reduced the rate at which NO disappeared.
Only a very limited number of papers studied the interaction of sulphur with NO in fuel-rich hydrocarbon flames have been published. In most cases, the effect reported was that of increasing NO concentrations by adding sulphur. Such is the case of the report by Corley and Wendt (Corley and Wendt (1984)) . Although the equivalence ratios studied were higher than those considered in this thesis ( = 1.74 and 2.18), they reported a large increases of NO concentrations from methane flames which used Ar as a diluent. Decreases of NO concentrations were observed only when Ar was replaced by He (N2 was the diluent in the drop tube furnace, which has similar heat transfer properties to He). However, the concentrations of CO2 were observed to rise with SO2, whereas they decreased in the drop tube furnace.
= 1.74 and 2.18), they reported a large increases of NO concentrations from methane flames which used Ar as a diluent. Decreases of NO concentrations were observed only when Ar was replaced by He (N2 was the diluent in the drop tube furnace, which has similar heat transfer properties to He). However, the concentrations of CO2 were observed to rise with SO2, whereas they decreased in the drop tube furnace.
Similar results obtained by other researchers were attributed to reactions of NO with reduced sulphur species such as SH (Chagger et al. (Chagger et al. (1991)) ) or a shift in the amine subsystem on reduction of radicals by SO2 (Chen et al. (Chen et al. (1984)) ).
Enhancement of NO2 in the presence of hydrocarbon species has been documented previously, and is discussed in section "5.1.5. Fuel-rich conditions ( = 1.200)" of chapter III. However, no previously published report has been found on the effect of SO2 on NO2 in fuel-rich, hydrocarbon systems. Two possible processes may be altered in order to cause the increment of NO2 concentrations measured (see Figure 149):
= 1.200)" of chapter III. However, no previously published report has been found on the effect of SO2 on NO2 in fuel-rich, hydrocarbon systems. Two possible processes may be altered in order to cause the increment of NO2 concentrations measured (see Figure 149):
 = 1.200 obtained concurrently to this experimental work (Molero and Burgess (1998)) showed a decrease of solid matter emissions with the addition of SO2, which confirms the existence of interactions between S and hydrocarbon species.
= 1.200 obtained concurrently to this experimental work (Molero and Burgess (1998)) showed a decrease of solid matter emissions with the addition of SO2, which confirms the existence of interactions between S and hydrocarbon species.
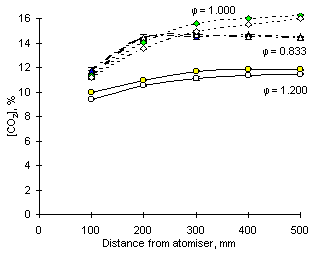
In stoichiometric and fuel-rich conditions the measured concentrations of CO2 decreased on addition of SO2, as can be observed in Figure 153. This may suggest that combustion is less complete in the presence of SO2. Reduced sulphur species and carbon monoxide may compete for oxidising compounds in this oxygen-deficient atmosphere. As the amounts of CO2 decrease, those of carbon monoxide and remaining hydrocarbons will therefore increase. It is also likely that the concentrations of hydroperoxyl radicals (RO2) will also rise, thus increasing the concentrations of NO2. However, more information about the process of NO2 formation by RO2 radicals must be known before a thorough rationale could be established. Although a number of papers have been published about this process at low temperature, no information is available at the temperatures found in combustion operation.
A practical application that could be derived from the enhancement of NO2 concentrations by sulphur is that it may facilitate NOX removal, as nitrogen dioxide can be removed by the same scrubbers that remove sulphur compounds from combustion gases.
 Previous |  Table of Contents |  Next |