5.2. SO2
A preliminary analysis of Figures 64 to 69 shows that the measured concentrations of SO2 rise with the equivalence ratio. These increases are due to the reduced volume of gases associated with the smaller addition of combustion air. Calculations of the amounts of fuel-S transformed into SO2 at  = 0.714 and 0.833 show that in both cases the conversion of fuel-S into SO2 was similar, at around 100 %.
= 0.714 and 0.833 show that in both cases the conversion of fuel-S into SO2 was similar, at around 100 %.
In fuel-lean conditions ( = 0.714 and 0.833) the formation and emission of SO2 seem to be independent of the furnace wall temperature. No increases were found when the furnace wall temperature was raised from 900 to 1,100 °C. With a large excess of oxygen available at these equivalence ratios, sulphur is likely to be fully oxidised to SO2, regardless the furnace wall temperature. In some cases the fuel-S conversion is slightly lower at
= 0.714 and 0.833) the formation and emission of SO2 seem to be independent of the furnace wall temperature. No increases were found when the furnace wall temperature was raised from 900 to 1,100 °C. With a large excess of oxygen available at these equivalence ratios, sulphur is likely to be fully oxidised to SO2, regardless the furnace wall temperature. In some cases the fuel-S conversion is slightly lower at  = 0.714 than at
= 0.714 than at  = 0.833, which may suggest the formation of SO3.
When the combustion mixture becomes fuel-rich the rate of formation of SO2 decreases. The shortage of oxygen at stoichiometric and fuel-rich conditions retards the formation of all SO2 until most O2 has been consumed. This can be seen in Figure 98.
= 0.833, which may suggest the formation of SO3.
When the combustion mixture becomes fuel-rich the rate of formation of SO2 decreases. The shortage of oxygen at stoichiometric and fuel-rich conditions retards the formation of all SO2 until most O2 has been consumed. This can be seen in Figure 98.
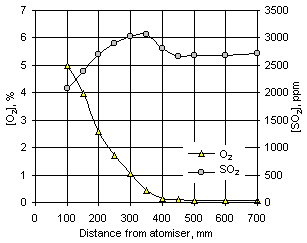
Figure 98: Formation of SO2 and disappearance of O2 for fuel M1 at  = 1.200 and 900 °C furnace wall temperature
= 1.200 and 900 °C furnace wall temperature
SO2 reaches a maximum and then decreases when approximately only 0.5 % O2 is left. Subsequently, the concentration of SO2 decreases as the remaining oxygen disappears. Larger decreases were found at higher furnace wall temperatures, as the peaks of SO2 concentration become more pronounced and the emissions lower. Results obtained by numerical modelling (see chapter VII for results of the numerical model) show that SO2 is reduced to species such as COS, SO and H2S in low-oxygen conditions. Furthermore, evidence was found in the drop-tube furnace of solid, yellow deposits, which can substantiate the formation of sulphur.
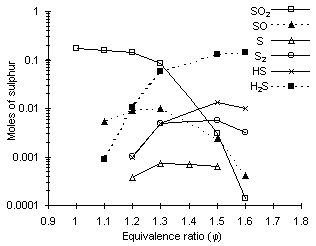
Figure 99: Equilibrium concentrations of sulphur species according to the equivalence ratio calculated using CHEMKIN (1.10 % wt sulphur, 1,527 °C)
A survey of the literature reveals that unlike in fuel-lean conditions, SO2 may not be the sole sulphur compound present in fuel-rich combustion environments. Other sulphur species such as H2S, S, S2, HS and SO may appear, as the relative importance of sulphur dioxide decreases. Figure 99 shows the calculated distribution of various predominant S compounds at various equivalence ratios (Graville (1993)) .
The reduction of sulphur dioxide can take place through the following fast bimolecular reactions (Cullis and Mulcahy (1972)) :
H + SO2  SO + OH SO + OH
| reac 92
|
H + SO  S + OH S + OH
| reac 93
|
S + H2  HS + H HS + H
| reac 94
|
HS + H2  H2S + H H2S + H
| reac 95
|
HS + HS  S2 + H2 S2 + H2
| reac 96
|
together with reactions of the radical pool:
O + H2  OH + H OH + H
| reac 5
|
H + O2  OH + O OH + O
| reac 6
|
H2 + OH  H + H2O H + H2O
| reac 7
|
Pollutant formation and interaction in the combustion of heavy liquid fuels
Luis Javier Molero de Blas, PhD thesis, University of London, 1998
© Luis Javier Molero de Blas
 = 0.714 and 0.833) the formation and emission of SO2 seem to be independent of the furnace wall temperature. No increases were found when the furnace wall temperature was raised from 900 to 1,100 °C. With a large excess of oxygen available at these equivalence ratios, sulphur is likely to be fully oxidised to SO2, regardless the furnace wall temperature. In some cases the fuel-S conversion is slightly lower at
= 0.714 and 0.833) the formation and emission of SO2 seem to be independent of the furnace wall temperature. No increases were found when the furnace wall temperature was raised from 900 to 1,100 °C. With a large excess of oxygen available at these equivalence ratios, sulphur is likely to be fully oxidised to SO2, regardless the furnace wall temperature. In some cases the fuel-S conversion is slightly lower at  = 0.714 than at
= 0.714 than at  = 0.833, which may suggest the formation of SO3.
When the combustion mixture becomes fuel-rich the rate of formation of SO2 decreases. The shortage of oxygen at stoichiometric and fuel-rich conditions retards the formation of all SO2 until most O2 has been consumed. This can be seen in Figure 98.
= 0.833, which may suggest the formation of SO3.
When the combustion mixture becomes fuel-rich the rate of formation of SO2 decreases. The shortage of oxygen at stoichiometric and fuel-rich conditions retards the formation of all SO2 until most O2 has been consumed. This can be seen in Figure 98.

 = 1.200 and 900 °C furnace wall temperature
= 1.200 and 900 °C furnace wall temperature
 SO + OH
SO + OH


