
An understanding of the fuel-NO formation process shows that changes in HCN concentrations engendered by sulphur would be of crucial importance. Investigations in moderately fuel-rich CH4/He/O2 flames doped with C2N2 were carried out by Corley and Wendt (Corley and Wendt (1984)). The fate of various nitrogenous species such as HCN, NO and N2 was monitored. Their results showed that concentrations of both NO and HCN were increased by the presence of SO2. This data suggested that SO2 could interact with the cyanide-amine subsystem that leads to the formation of fuel-NO.
On the contrary, concentrations of N2 decreased when SO2 was present. A discrepancy in the mass balance of nitrogen led to the conclusion that some N2O was formed from the initial nitrogenous species, which was also postulated by Tseregounis and Smith (Tseregounis and Smith (1984)) (see next paragraphs).
Pfefferle and Churchill (Pfefferle and Churchill (1989)) also investigated the effect of fuel-S on nitrogenous species. Combustion of ethane, air, NH3 (fuel-N) and H2S (fuel-S) in a Thermally Stabilised Burner (TSB) was forced by a hot surface, as detailed in previous paragraphs on thermal-NOX. Again the "sparse" numerical model, comprising a variety of sulphur reactions (oxidation, reactions of SO2 and SO3 with atomic oxygen, sulphur-nitrogen and sulphur-carbon) in a simulated TSB burner, was employed in the calculations. Fuel-NOX emissions were decreased by H2S between 10 and 20 %, which was greater than the reduction of thermal-NOX. Results from the numerical model ruled out competition for the available oxidant as the only source of NOX decrease, whilst indirect radical rearrangements were also thought to play an important role.
As opposed to work by other authors, the numerical model performed by Pfefferle and Churchill demonstrated the importance of the reactions in which the NS was involved. Inclusion of these reactions (numbers 259 through to 261 in "Appendix III" of this thesis) yielded a better agreement between experimental and numerical results due to their action to reduce NO to N2, especially at fuel-rich equivalence ratios, where formation of NS is favoured. However, the reductions of NOX predicted by the model were lower than those registered experimentally. The researchers concluded that key reactions in the N-S interaction processes were still missing from the model, although it was successful at predicting diminished concentrations of O and OH radicals.
Experimental evidence of the existence of substantial quantities of NS radicals have been found in the flame front of fuel-rich methane and natural gas flames doped with NH3 and H2S or SF6. Jeffries and Crosley (Jeffries and Crosley (1986)) found the concentration of NS radicals to be relatively high at approximately 0.1 % of that of H2S added to the flame in most cases, depending on the oxidiser. NS concentration was in steady state as a result of rapid production and recombination processes in the flame front. Formation reactions were thought to be those of NO and NHi radicals with reduced S species such as CS2, S2, H2S and SH. NHi radicals were also important in recombination reactions with NS, as well as oxidation reactions with NO, O2, O, OH, N2O and NO2. Other possible direct reactions between sulphur and nitrogenous species have also been investigated. Wendt and Sternling (Wendt and Sternling (1973)) performed computer calculations of the oxidation of SO2 to SO3 via NO catalysis through the following mechanism (Armitage and Cullis (1971), Cullis et al. (1966)):
NO + O2  NO3 NO3
| reac 74 |
NO3 + SO2  SO3 + NO2 SO3 + NO2
| reac 75 |
However, they found that at NO concentrations typical of post-flame stack gases SO3 was formed in negligible amounts via this mechanism.
The existence of NS radicals has been contemplated in previous paragraphs. Other sulphurous species are also present in combustion systems. Sulphur normally exists in the fuel in its reduced state as H2S, but Zachariah and Smith (Zachariah and Smith (1987)) found a variety of sulphur species formed in fuel-rich H2/O2/SO2 flames. SO2 was the predominant compound, but SO was also formed in the reaction zone of the flame. Subsequent decay of its concentration leads to renewed formation of reduced species: H2S, SH and S2, the latter being the most important species after SO2. At long residence times H2S becomes the most important species, with concentrations higher than those of SO2.
One possible path for NO reduction in a post-flame situation is the recycling process with CH and CH2 to give place to HCN (see section "4.1.1.c. Oxidation of fuel-bound nitrogen compounds: Fuel-NO"). Can this path be affected by sulphur compounds? Work by Tseregounis and Smith (Tseregounis and Smith (1983)) revealed large increases in fuel-NO formation when SO2 (gas) was added to premixed, laminar, fuel-rich H2/C2H2 flames doped with C2N2 to simulate fuel-N. The results from H2 flames (thus absence of hydrocarbons) ruled out the postulate that the increase of NO concentrations was due to inhibition of the recycling process between NO and hydrocarbons by the addition of a sulphur compound. In addition, their calculations showed that direct interactions between fuel-N and fuel-S of the kind
 NO + S reac 76
NO + S reac 76were not plausible as this would have led to a decrease in NO concentrations in fuel-rich conditions. The relatively low importance of this kind of direct interactions was also assessed by Chen et al. (Chen et al. (1984)), as reported in following paragraphs.
However, opposite conclusions were obtained experimentally and numerically by Wendt et al. (Wendt et al. (1983)) in studies of rich, moist, CO/Ar/O2 flames doped with C2N2. The absence of H radicals ruled out the potential role of radical recombination as a NOX reduction means in this case. Although the results could not be extrapolated to hydrocarbon flames, the authors attributed reductions of NO in the post-flame zone to the action of the reverse Zeldovich mechanism only:
 N2 + O reac -2
N2 + O reac -2the N radicals being formed in:
 N + O2 reac -3
N + O2 reac -3Direct interactions of sulphur species with nitrogenous species were ascribed for accelerated reductions of NO and N2 formation by means of:
N + SO  NO + S NO + S
| reac 76 |
NO + S  NS + O NS + O
| reac 77 |
NS + O  SO + N SO + N
| reac 78 |
Their work was continued by Corley and Wendt (Corley and Wendt (1984)) with rich CH4/He/O2 flames doped with C2N2 and SO2. Contrary to results with the simpler CO flames, greatly increased concentrations of NO were found at  = 1.71. HCN also increased, whereas N2 decreased on addition of SO2. Increased NO concentrations were considered to be caused by the direct interaction of N and SO radicals in reaction 3. This reaction was complemented by other interactions of SO2 and its reduced species in the cyanide and amine subsystems, such as:
= 1.71. HCN also increased, whereas N2 decreased on addition of SO2. Increased NO concentrations were considered to be caused by the direct interaction of N and SO radicals in reaction 3. This reaction was complemented by other interactions of SO2 and its reduced species in the cyanide and amine subsystems, such as:
CN + SO  NCO + S NCO + S
| reac 79 |
NH + SO  HNO + S HNO + S
| reac 80 |
Corley and Wendt (Corley and Wendt (1984)) also found that the addition of SO2 decreased the amounts of soot formed at fuel-rich conditions, and that this soot contained some nitrogenous compounds. Interaction between the soot formation mechanism, SO2 and nitrogenous compounds was thus postulated.
Interactions of SO2 with the mechanism of soot formation were also observed by Lawton (Lawton (1989)). Significant decreases of the concentration of polycyclic aromatic hydrocarbons (PAH's) were caused by the action of SO2 in rich-premixed ethylene flames. By interaction with the soot formation processes, sulphur dioxide can disappear and remove carbon to give place to other species such as CO, CO2, COS and CS2. These species may enter other reaction channels.
Detailed work performed by Tseregounis and Smith (Tseregounis and Smith (1984)) showed that SO2 decreased the concentrations of most radicals (O, OH and H) by almost 50 % in fuel-rich H2/O2/Ar flames. The dopants were C2N2 and SO2. The highest reductions took place in near-stoichiometric conditions. Conversely, the profiles of HCN were not greatly changed with addition of SO2. The overall effect of SO2 addition on fuel-NO emissions was an increase of its formation rate in the initial stages, followed by a decrease in its consumption in the downstream regions of the flame. Thus, increases of fuel-NO were observed again. Tseregounis and Smith suggested a mechanism of NO reduction in the post-flame zone which, because of its dependence on H radical concentrations, leads to the increase of NO emissions by SO2:
NO + H + M  HNO + M HNO + M
| reac 81 |
HNO + H  NH + OH NH + OH
| reac 82 |
HNO + OH  NO + H2O NO + H2O
| reac 83 |
NH + NO  N2 + OH N2 + OH
| reac 84 |
together with the H radical recombination by SO2:
H + SO2 + M  HSO2 + M HSO2 + M
| reac 62 |
H + HSO2  SO2 + H2 SO2 + H2
| reac 63 |
Tseregounis and Smith observed increases in the concentration of HCN only at slightly fuel-rich conditions, and no changes at other equivalence ratios. Increased HCN concentrations would decrease NO concentrations, which was not the effect observed.
The fact that radical concentrations are lowered by the presence of sulphur was also observed in fuel-rich hydrogen flames by Chen et al. (Chen et al. (1984)). In a variety of equivalence ratios (mostly fuel-rich) the concentrations of H radicals were seen to decrease on addition of SO2, thus lowering the formation of NH and N from NO via HNO. The reduction mechanism was similar to that reported by Tseregounis and Smith (Tseregounis and Smith (1984)), and was extended to incorporate reactions of amine species:
NO + H + M  HNO + M HNO + M
| reac 81 |
NO + N  N2 + O N2 + O
| reac -2 |
HNO + H  NH + OH NH + OH
| reac 82 |
HNO + OH  NO + H2O NO + H2O
| reac 83 |
HNO + H  NO + H2 NO + H2
| reac 85 |
NH + H  N + H2 N + H2
| reac 19 |
NH + OH  N + H2O N + H2O
| reac 86 |
NH + NO  N2 + OH N2 + OH
| reac 87 |
Thus lower H concentrations caused a decrease in the extent of NO reduction, which would give rise to higher NO emissions.
When experiments were carried out by Chen et al. in propane flames in a jet stirred reactor, increases of NH3 and HCN with the addition of SO2 were reported which led to increased NO emissions. In relatively complex flames such as this it is likely that the processes for NO reduction will be different according to the flame zone considered, which is a concept that was also incorporated by Graville (Graville (1993)). Chen et al. acknowledged four major routes for the reaction of NO in hydrocarbon flames:
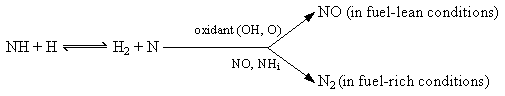
 HCN + ...) which is predominant in the injection zone, where hydrocarbon concentrations are high (prompt-NO)
HCN + ...) which is predominant in the injection zone, where hydrocarbon concentrations are high (prompt-NO)
 NO + H reac 4
NO + H reac 4Wendt et al. (Wendt et al. (1979)) acknowledged that sulphur can change the formation of NO substantially in fuel-rich environments. They studied the dependence of such a change on local stoichiometry, as local fuel-rich conditions present in many realistic combustion systems are likely to promote N-S interaction. Combustion air was pre-heated and the fuel was methane.
Increases of NO concentrations were obtained near the burner front, whereas a gradual decrease was caused by S as the residence time became longer. Wendt et al. (Wendt et al. (1979)) attributed the change in NO profiles to two different effects: Its initial enhancement may be due to reaction 76:
 NO + S reac 76
NO + S reac 76A subsequent reduction is caused by NHX species in reactions such as -2, 39 and 87. Although amine species are expected to disappear shortly after the flame front, the presence of sulphur may extend their lifetime. This was also observed by Tseregounis and Smith (Tseregounis and Smith (1983)).
Wendt et al. (Wendt et al. (1979)) also investigated the effect of local stoichiometry in turbulent diffusion flames of distillate oil. Various forms of sulphur dopant caused a 40 % increase of NO as long as mixing was poor. In high swirl flames (good air-fuel mixing, therefore no fuel-rich conditions present) sulphur had no effect on emissions of nitrogen oxides. The stoichiometry of the combustion mixture was also an important parameter in the work performed by Tang et al. (Tang et al. (1981)) in a refractory burner. Hexane was doped with varying amounts of tertiary butyl mercaptan and pyridine in order to simulate fuel-S and fuel-N, respectively. Experiments were performed at fuel-lean, stoichiometric and fuel-rich equivalence ratios. NOX formed from pyridine was reduced by sulphur at all equivalence ratios, but the extent of the reduction was largely independent of the amounts of sulphur added.
On the contrary, recent work by Hampartsoumian and Nimmo (Hampartsoumian and Nimmo (1995)) showed that fuel-NO could be increased with the concentration of sulphur in the flame at all combustion stoichiometries. A nearly linear increase of fuel-NO was found when increasing amounts of S were added to flames from a variety of complex fuels. Conversely, it was also found that the increase of fuel-NO emissions caused by sulphur was directly proportional to the amount of N in fuel. In addition, and similar to the results reported by Wendt et al. (Wendt et al. (1979)), staging of the combustion air had a strong effect on fuel-NO as a more pronounced NO enhancement was observed when the conditions in the primary zone became fuel-rich. Reactions of sulphur with N species have been proposed to explain the increase of fuel-NO caused by fuel-sulphur:
N + SO  NO + S NO + S
| reac 76 |
NO + S  NS + O NS + O
| reac 77 |
NS + O  SO + N SO + N
| reac 78 |
SH + NH  NS + H2 NS + H2
| reac 88 |
N + NS  N2 + S N2 + S
| reac 89 |
N + SH  NS + H NS + H
| reac 90 |
the latter three being able to remove amine species from NHi-NO reduction reactions like:
NH2 + NO  N2 + H2O N2 + H2O
| reac 39 |
NH + NO  N2 + OH N2 + OH
| reac 87 |
N + NO  N2 + O N2 + O
| reac -2 |
which in turn may lead to higher NO concentrations. Graville (Graville (1993)) published the only report on N-S interactions in heavy fuel oils to date. These fuels contain large amounts of sulphur and nitrogen, the latter usually in the form of asphaltenes of high boiling point. The release of nitrogen and sulphur from these compounds is thus gradual, and they can enter the NOX formation or reduction processes at different stages, normally later than volatile nitrogen compounds. This feature has a strong potential for achieving large NOX reductions, and also shows the importance of the residence time in these processes. In his experimental work, Graville reported reductions of NOX emissions between 20 and 30 % on addition of 1 % S as thianthrene (fuel-S) to a residual fuel oil. The efficacy of sulphur as a NOX suppresser was diminished as the fuel-N content increased. Conversely, addition of light N and S additives to a hydrogenated gas oil did not cause change of NOX concentrations. The early release of N and S from this light fuel could not contribute to the NOX reduction processes in the postflame zone. His results, which were partly predicted by means of numerical modelling, provided supporting evidence for the competition of oxygen-containing radicals at long residence times, resulting in a decrease of NOX emissions. Turbulence effects were also considered of importance as they can influence the distribution of radicals.
The situation is rather more confusing with respect to fuel-NOX. Increases and reductions of fuel-NOX have been found mainly in fuel-rich conditions, although other equivalence ratios have also been studied. A variety of mechanisms have been proposed, such as direct N-S interactions, radical rearrangements, interference with NOX-hydrocarbon reactions or the amine subsystem... In addition, the recombination of radicals by the presence of sulphur species can affect the reduction of nitric oxide after its formation.
The need for further work to clarify the array of possible routes for the interaction of NOX and SOX compounds originated from the fuel is thus clear. The experimental work carried out on the topic in this thesis is reported in chapter VII.
 Previous |  Table of Contents |  Next |