Elements:
C H N O S
Species:
H2 H2O HO2 OH CH CH2 CH2(S) CH3 CH4 C2H C2H2 C2H3 C2H4 C2H5 C2H6
C3H2 C3H3 C4H2 C4H3 HCO CH2O CH3O HCCO HCCOH CH2CO CH2OH CO
CO2 O2 HCNO HNCO HOCN N2 NNH NH2 NH3 NO NO2 N2O HNO H2O2 HCN
H2CN NH CN C2N2 NCO COS CS SO SO2 SO3 SH H2S HSO2 SN
CH2(S) stands for the singlet form of CH2.
The Sandia thermodynamic data-base does not include reaction rate coefficients for HSO2. They are necessary to calculate the various thermodynamic parameters used in the model. Such data was taken from Zachariah and Smith (Zachariah and Smith (1987)), and their values are:
A1 = 4.081
A2 = 8.375×10-3
A3 = -4.722×10-6
A4 = 9.349×10-10
A5 = 0
A6 = -31.56
A7 = 8.610
Reactions:
The reactions included in the mechanism are listed below.
The mechanism described includes some reactions with third-body efficiency (Graville (1993)) , denoted by the inclusion of a so-called M reactant. For such reactions lines are included that identify the elements that may act as third bodies. Their corresponding efficiency coefficients are given.
The Arrhenius parameters of the reactions were provided for calculation of the reaction rate coeffcients by the numerical simulation package in the form:
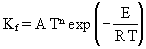
In the case of reactions 254, 255 and 256, different values of the Arrhenius parameters have been published. Since CHEMKIN allows the use of both sets of values, duplicate reactions have been declared.
Reaction no. Reaction Pre-exp. factor T exp. Act. energy, (mol/cm3 K) n (cal/mol) 1. CH3+CH3=C2H6 3.16E+36 -7.2 750.0 2. CH3+H=CH4 2.08E+25 -4.0 665.0 3. CH4+O2=CH3+HO2 7.90E+13 0.0 56000.0 4. CH4+H=CH3+H2 2.20E+04 3.0 8750.0 5. CH4+OH=CH3+H2O 1.60E+06 2.1 2460.0 6. CH4+O=CH3+OH 1.02E+09 1.5 8604.0 7. CH4+HO2=CH3+H2O2 1.80E+11 0.0 18700.0 8. CH3+HO2=CH3O+OH 2.00E+13 0.0 0.0 9. CH3+O2=CH3O+O 2.05E+19 -1.57 29229.0 10. CH3+O=CH2+OH 8.00E+13 0.0 0.0 11. CH2OH+H=CH3+OH 1.00E+14 0.0 0.0 12. CH3O+H=CH3+OH 1.00E+14 0.0 0.0 13. CH3+OH=CH2+H2O 7.50E+06 2.0 5000.0 14. CH3+H=CH2+H2 9.00E+13 0.0 15100.0 15. CH3O+M=CH2O+H+M 1.00E+14 0.0 25000.0 16. CH2OH+M=CH2O+H+M 1.00E+14 0.0 25000.0 17. CH3O+H=CH2O+H2 2.00E+14 0.0 0.0 18. CH2OH+H=CH2O+H2 2.00E+13 0.0 0.0 19. CH3O+OH=CH2O+H2O 1.00E+13 0.0 0.0 20. CH2OH+OH=CH2O+H2O 1.00E+13 0.0 0.0 21. CH3O+O=CH2O+OH 1.00E+13 0.0 0.0 22. CH2OH+O=CH2O+OH 1.00E+13 0.0 0.0 23. CH3O+O2=CH2O+HO2 6.30E+10 0.0 2600.0 24. CH2OH+O2=CH2O+HO2 1.48E+13 0.0 1500.0 25. CH2+H=CH+H2 1.00E+18 -1.56 0.0 26. CH2+OH=CH+H2O 1.13E+07 2.0 3000.0 27. CH2+OH=CH2O+H 2.50E+13 0.0 0.0 28. CH+O2=HCO+O 3.30E+13 0.0 0.0 29. CH+O=CO+H 5.70E+13 0.0 0.0 30. CH+OH=HCO+H 3.00E+13 0.0 0.0 31. CH+CO2=HCO+CO 3.40E+12 0.0 690.0 32. CH+H=C+H2 1.50E+14 0.0 0.0 33. CH+H2O=CH2O+H 1.17E+15 -0.75 0.0 34. CH+CH2O=CH2CO+H 9.46E+13 0.0 -515.0 35. CH+C2H2=C3H2+H 1.00E+14 0.0 0.0 36. CH+CH2=C2H2+H 4.00E+13 0.0 0.0 37. CH+CH3=C2H3+H 3.00E+13 0.0 0.0 38. CH+CH4=C2H4+H 6.00E+13 0.0 0.0 39. C+O2=CO+O 2.00E+13 0.0 0.0 40. C+OH=CO+H 5.00E+13 0.0 0.0 41. C+CH3=C2H2+H 5.00E+13 0.0 0.0 42. C+CH2=C2H+H 5.00E+13 0.0 0.0 43. CH2+CO2=CH2O+CO 1.10E+11 0.0 1000.0 43. CH2+CO2=CH2O+CO 1.10E+11 0.0 1000.0 44. CH2+O=CO+H+H 5.00E+13 0.0 0.0 45. CH2+O=CO+H2 3.00E+13 0.0 0.0 46. CH2+O2=CO2+H+H 1.60E+12 0.0 1000.0 47. CH2+O2=CH2O+O 5.00E+13 0.0 9000.0 48. CH2+O2=CO2+H2 8.60E+10 0.0 -500.0 49. CH2+O2=HCO+OH 4.30E+10 0.0 -500.0 50. CH2O+OH=HCO+H2O 3.43E+09 1.18 -447.0 51. CH2O+H=HCO+H2 2.19E+08 1.77 3000.0 52. CH2O+M=HCO+H+M 3.31E+16 0.0 81000.0 53. CH2O+O=HCO+OH 1.80E+13 0.0 3080.0 54. HCO+OH=H2O+CO 1.00E+14 0.0 0.0 55. HCO+M=H+CO+M 2.50E+14 0.0 16802.0 CO enhanced by 1.9 H2 enhanced by 1.9 CH4 enhanced by 2.8 CO2 enhanced by 3.0 H2O enhanced by 5.0 56. HCO+H=CO+H2 1.19E+13 0.25 0.0 57. HCO+O=CO+OH 3.00E+13 0.0 0.0 58. HCO+O=CO2+H 3.00E+13 0.0 0.0 59. HCO+O2=HO2+CO 3.30E+13 -0.4 0.0 60. CO+O+M=CO2+M 6.17E+14 0.0 3000.0 61. CO+OH=CO2+H 1.51E+07 1.3 -758.0 62. CO+O2=CO2+O 1.60E+13 0.0 41000.0 63. HO2+CO=CO2+OH 5.80E+13 0.0 22934.0 64. C2H6+CH3=C2H5+CH4 5.50E-01 4.0 8300.00 65. C2H6+H=C2H5+H2 5.40E+02 3.5 5210.0 66. C2H6+O=C2H5+OH 3.00E+07 2.0 5115.0 67. C2H6+OH=C2H5+H2O 8.70E+09 1.05 1810.0 68. C2H4+H=C2H3+H2 1.10E+14 0.0 8500.0 69. C2H4+O=CH3+HCO 1.60E+09 1.2 746.0 70. C2H4+OH=C2H3+H2O 2.02E+13 0.0 5955.0 71. CH2+CH3=C2H4+H 3.00E+13 0.0 0.0 72. H+C2H4=C2H5 3.52E+18 -1.5 5305.0 73. C2H5+H=CH3+CH3 1.00E+14 0.0 0.0 74. C2H5+O2=C2H4+HO2 8.43E+11 0.0 3875.0 75. C2H2+O=CH2+CO 1.02E+07 2.0 1900.0 76. C2H2+O=HCCO+H 1.02E+07 2.0 1900.0 77. H2+C2H=C2H2+H 4.09E+05 2.39 864.0 78. H+C2H2=C2H3 1.55E+30 -5.68 6810.0 79. C2H3+H=C2H2+H2 4.00E+13 0.0 0.0 80. C2H3+O=CH2CO+H 3.00E+13 0.0 0.0 81. C2H3+O2=CH2O+HCO 4.00E+12 0.0 -250.0 82. C2H3+OH=C2H2+H2O 5.00E+12 0.0 0.0 83. C2H3+CH2=C2H2+CH3 3.00E+13 0.0 0.0 84. C2H3+C2H=C2H2+C2H2 3.00E+13 0.0 0.0 85. C2H3+CH=CH2+C2H2 5.00E+13 0.0 0.0 86. OH+C2H2=C2H+H2O 3.37E+07 2.0 14000.0 87. OH+C2H2=HCCOH+H 5.04E+05 2.3 13500.0 88. OH+C2H2=CH2CO+H 2.18E-04 4.5 -1000.0 89. OH+C2H2=CH3+CO 4.83E-04 4.0 -2000.0 90. HCCOH+H=CH2CO+H 1.00E+13 0.0 0.0 91. C2H2+O=C2H+OH 3.16E+15 0.6 15000.0 92. CH2CO+O=CO2+CH2 1.75E+12 0.0 1350.0 93. CH2CO+H=CH3+CO 1.13E+13 0.0 3428.0 94. CH2CO+H=HCCO+H2 5.00E+13 0.0 8000.0 95. CH2CO+O=HCCO+OH 1.00E+13 0.0 8000.0 96. CH2CO+OH=HCCO+H2O 7.50E+12 0.0 2000.0 97. CH2CO=CH2+CO 1.20E+28 -4.99 70780.0 98. C2H+O2=CO+CO+H 5.00E+13 0.0 1500.0 99. C2H+C2H2=C4H2+H 3.00E+13 0.0 0.0 100. H+HCCO=CH2(S)+CO 1.00E+14 0.0 0.0 101. O+HCCO=H+CO+CO 1.00E+14 0.0 0.0 102. HCCO+O2=CO+CO+OH 1.60E+12 0.0 854.0 103. CH+HCCO=C2H2+CO 5.00E+13 0.0 0.0 104. HCCO+HCCO=C2H2+CO+CO 1.00E+13 0.0 0.0 105. CH2(S)+M=CH2+M 1.00E+13 0.0 0.0 H enhanced by 0.0 106. CH2(S)+CH4=CH3+CH3 4.00E+13 0.0 0.0 107. CH2(S)+C2H6=CH3+C2H5 1.20E+14 0.0 0.0 108. CH2(S)+O2=CO+OH+H 3.00E+13 0.0 0.0 109. CH2(S)+H2=CH3+H 7.00E+13 0.0 0.0 110. CH2(S)+H=CH2+H 2.00E+14 0.0 0.0 111. C2H+O=CH+CO 5.00E+13 0.0 0.0 112. C2H+OH=HCCO+H 2.00E+13 0.0 0.0 113. CH2+CH2=C2H2+H2 4.00E+13 0.0 0.0 114. CH2+HCCO=C2H3+CO 3.00E+13 0.0 0.0 115. CH2+C2H2=C3H3+H 1.20E+13 0.0 6600.0 116. C4H2+OH=C3H2+HCO 6.66E+12 0.0 -410. 117. C3H2+O2=HCO+HCCO 1.00E+13 0.0 0.0 118. C3H3+O2=CH2CO+HCO 3.00E+10 0.0 2868.0 119. C3H3+O=CH2O+C2H 2.00E+13 0.0 0.0 120. C3H3+OH=C3H2+H2O 2.00E+13 0.0 0.0 121. C2H2+C2H2=C4H3+H 2.00E+12 0.0 45900.0 122. C4H3+M=C4H2+H+M 1.00E+16 0.0 59700.0 123. CH2(S)+C2H2=C3H3+H 3.00E+13 0.0 0.0 124. C4H2+O=C3H2+CO 1.20E+12 0.0 0.0 125. C2H2+O2=HCCO+OH 2.00E+08 1.5 30100.0 126. C2H2+M=C2H+H+M 4.20E+16 0.0 107000.0 127. C2H4+M=C2H2+H2+M 1.50E+15 0.0 55800.0 128. C2H4+M=C2H3+H+M 1.40E+16 0.0 82360.0 129. H2+O2=OH+OH 1.70E+13 0.0 47780.0 130. OH+H2=H2O+H 1.17E+09 1.3 3626.0 131. O+OH=O2+H 4.00E+14 -0.5 0 132. O+H2=OH+H 5.06E+04 2.67 6290.0 133. H+O2+M=HO2+M 3.61E+17 -0.72 0.0 H2O enhanced by 1.86 CO2 enhanced by 4.2 H2 enhanced by 2.9 CO enhanced by 2.1 N2 enhanced by 1.3 134. OH+HO2=H2O+O2 7.50E+12 0.0 0.0 135. H+HO2=OH+OH 1.40E+14 0.0 1073.0 136. O+HO2=O2+OH 1.40E+13 0.0 1073.0 137. OH+OH=O+H2O 6.00E+08 1.3 0.0 138. H+H+M=H2+M 1.00E+18 -1.0 0.0 H2 enhanced by 0.0 H2O enhanced by 0.0 CO2 enhanced by 0.0 139. H+H+H2=H2+H2 9.20E+16 -0.6 0.0 140. H+H+H2O=H2+H2O 6.00E+19 -1.25 0.0 141. H+H+CO2=H2+CO2 5.49E+20 -2.0 0.0 142. H+OH+M=H2O+M 1.60E+22 -2.0 0.0 H2O enhanced by 5.0 143. H+O+M=OH+M 6.20E+16 -0.6 0.0 H2O enhanced by 5.0 144. O+O+M=O2+M 1.89E+13 0.0 -1788.0 145. H+HO2=H2+O2 1.25E+13 0.0 0.0 146. HO2+HO2=H2O2+O2 2.00E+12 0.0 0.0 147. H2O2+M=OH+OH+M 1.30E+17 0.0 45500.0 148. H2O2+H=HO2+H2 1.60E+12 0.0 3800.0 149. H2O2+OH=H2O+HO2 1.00E+13 0.0 1800.0 150. CH+N2=HCN+N 3.00E+11 0.0 13600.0 151. CN+N=C+N2 1.04E+15 -0.5 0.0 152. CH2+N2=HCN+NH 1.00E+13 0.0 74000.0 153. H2CN+N=N2+CH2 2.00E+13 0.0 0.0 154. H2CN+M=HCN+H+M 3.00E+14 0.0 22000.0 155. C+NO=CN+O 6.60E+13 0.0 0.0 156. CH+NO=HCN+O 1.10E+14 0.0 0.0 157. CH2+NO=HCNO+H 1.39E+12 0.0 -1100.0 158. CH3+NO=HCN+H2O 1.00E+11 0.0 15000.0 159. CH3+NO=H2CN+OH 1.00E+11 0.0 15000.0 160. HCCO+NO=HCNO+CO 2.00E+03 0.0 0.0 161 CH2(S)+NO=HCN+OH 2.00E+13 0.0 0.0 162. HCNO+H=HCN+OH 1.00E+14 0.0 12000.0 163. CH2+N=HCN+H 5.00E+13 0.0 0.0 164. CH+N=CN+H 1.30E+13 0.0 0.0 165. CO2+N=NO+CO 1.90E+11 0.0 3400.0 166. HCCO+N=HCN+CO 5.00E+13 0.0 0.0 167. CH3+N=H2CN+H 3.00E+13 0.0 0.0 168. C2H3+N=HCN+CH2 2.00E+13 0.0 0.0 169. C3H3+N=HCN+C2H2 1.00E+13 0.0 0.0 170. HCN+OH=CN+H2O 1.45E+13 0.0 10929.0 171. OH+HCN=HOCN+H 5.85E+04 2.4 12500.0 172. OH+HCN=HNCO+H 1.98E-03 4.0 1000.0 173. OH+HCN=NH2+CO 7.83E-04 4.0 4000.0 174. HOCN+H=HNCO+H 1.00E+13 0.0 0.0 175. HCN+O=NCO+H 1.38E+04 2.64 4980.0 176. HCN+O=NH+CO 3.45E+03 2.64 4980.0 177. HCN+O=CN+OH 2.70E+00 1.58 26600.0 178. CN+H2=HCN+H 2.95E+05 2.45 2237.0 179. CN+O=CO+N 1.80E+13 0.0 0.0 180. CN+O2=NCO+O 5.60E+12 0.0 0.0 181. CN+OH=NCO+H 6.00E+13 0.0 0.0 182. CN+HCN=C2N2+H 2.00E+13 0.0 0.0 183. CN+NO2=NCO+NO 3.00E+13 0.0 0.0 184. CN+N2O=NCO+N2 1.00E+13 0.0 0.0 185. C2N2+O=NCO+CN 4.57E+12 0.0 8880.0 186. C2N2+OH=HOCN+CN 1.86E+11 0.0 2900.0 187. HO2+NO=NO2+OH 2.11E+12 0.0 -479.0 188. NO2+H=NO+OH 3.50E+14 0.0 1500.0 189. NO2+O=NO+O2 1.00E+13 0.0 600.0 190. NO2+M=NO+O+M 1.10E+16 0.0 66000.0 191. NCO+H=NH+CO 5.00E+13 0.0 0.0 192. NCO+O=NO+CO 2.00E+13 0.0 0.0 193. NCO+N=N2+CO 2.00E+13 0.0 0.0 194. NCO+OH=NO+CO+H 1.00E+13 0.0 0.0 195. NCO+M=N+CO+M 3.10E+16 -0.5 48000.0 196. NCO+NO=N2O+CO 1.00E+13 0.0 -390.0 197. NCO+H2=HNCO+H 8.58E+12 0.0 9000.0 198. HNCO+H=NH2+CO 2.00E+13 0.0 3000.0 199. NH+O2=HNO+O 1.00E+13 0.0 12000.0 200. NH+O2=NO+OH 7.60E+10 0.0 1530.0 201. NH+NO=N2O+H 2.40E+15 -0.8 0.0 202. N2O+OH=N2+HO2 2.00E+12 0.0 10000.0 203. N2O+H=N2+OH 7.60E+13 0.0 15200.0 204. N2O+M=N2+O+M 1.60E+14 0.0 51600.0 205. N2O+O=N2+O2 1.00E+14 0.0 28200.0 206. N2O+O=NO+NO 1.00E+14 0.0 28200.0 207. NH+OH=HNO+H 2.00E+13 0.0 0.0 208. NH+OH=N+H2O 5.00E+11 0.5 2000.0 209. NH+N=N2+H 3.00E+13 0.0 0.0 210. NH+H=N+H2 1.00E+14 0.0 0.0 211. NH2+O=HNO+H 6.63E+14 -0.5 0.0 212. NH2+O=NH+OH 6.75E+12 0.0 0.0 213. NH2+OH=NH+H2O 4.00E+06 2.0 1000.0 214. NH2+H=NH+H2 6.92E+13 0.0 3650.0 215. NH2+NO=NNH+OH 6.40E+15 -1.25 0.0 216. NH2+NO=N2+H2O 6.20E+15 -1.25 0.0 217. NH3+OH=NH2+H2O 2.04E+06 2.04 566.0 218. NH3+H=NH2+H2 6.36E+05 2.39 10171.0 219. NH3+O=NH2+OH 2.10E+13 0.0 9000.0 220. NNH=N2+H 1.00E+04 0.0 0.0 221. NNH+NO=N2+HNO 5.00E+13 0.0 0.0 222. NNH+H=N2+H2 1.00E+14 0.0 0.0 223. NNH+OH=N2+H2O 5.00E+13 0.0 0.0 224. NNH+NH2=N2+NH3 5.00E+13 0.0 0.0 225. NNH+NH=N2+NH2 5.00E+13 0.0 0.0 226. NNH+O=N2O+H 1.00E+14 0.0 0.0 227. HNO+M=H+NO+M 1.50E+16 0.0 48680.0 H2O enhanced by 10.0 O2 enhanced by 2.0 N2 enhanced by 2.0 H2 enhanced by 2.0 228. HNO+OH=NO+H2O 3.60E+13 0.0 0.0 229. HNO+H=H2+NO 5.00E+12 0.0 0.0 230. HNO+NH2=NH3+NO 2.00E+13 0.0 1000.0 231. N+NO=N2+O 3.27E+12 0.3 0.0 232. N+O2=NO+O 6.40E+09 1.0 6280.0 233. N+OH=NO+H 3.80E+13 0.0 0.0 234. SO3+O=SO2+O2 6.40E+14 0.0 10800.0 235. SO2+O+M=SO3+M 1.00E+17 0.0 0.0 236. SO2+NO2=NO+SO3 1.00E+13 0.0 27000.0 237. SO+O2=SO2+O 4.00E+15 0.0 0.0 238. SO+OH=SO2+H 1.00E+14 0.0 0.0 239. SO+NO2=SO2+NO 1.00E+13 0.0 0.0 240. SO+O+M=SO2+M 1.00E+18 0.0 0.0 241. SO+N=NO+S 5.10E+11 0.0 0.0 242. SO+SO=SO2+S 1.00E+11 0.0 0.0 243. SH+O2=OH+SO 1.00E+14 0.0 0.0 244. COS+O=CO+SO 6.60E+12 0.0 0.0 245. S+O2=SO+O 1.00E+13 0.0 5600.0 246. SH+H2=H2S+H 1.80E+13 0.0 600.0 247. SH+O=SO+H 3.00E+14 0.0 0.0 248. OH+H2S=H2O+SH 1.40E+13 0.0 900.0 249. O+H2S=OH+SH 4.00E+12 0.0 3290.0 250. S+H2=SH+H 6.00E+13 0.0 0.0 251. S+OH=SH+O 3.00E+13 0.0 0.0 252. S+H2O=SH+OH 4.30E+12 0.0 37000.0 253. H+SO3=OH+SO2 6.00E+12 0.0 0.0 254-1. H+SO2+M=HSO2+M 1.40E+06 0.0 0.0 254-2. H+SO2+M=HSO2+M 7.30E+16 0.0 0.0 255-1. H+HSO2=H2+SO2 1.00E+12 0.0 0.0 255-2. H+HSO2=H2+SO2 1.60E+12 0.0 0.0 256-1. OH+HSO2=H2O+SO2 1.00E+12 0.0 0.0 256-2. OH+HSO2=H2O+SO2 6.80E+13 0.0 1772.0 257. S+NH=SH+N 1.00E+13 0.0 0.0 258. NH+SO=NO+SH 1.00E+13 0.0 0.0 259. SH+NH=SN+H2 1.00E+13 0.0 0.0 260. SN+NO=N2+SO 1.00E+13 0.0 0.0 261. NO+CS=SN+CO 1.00E+13 0.0 0.0 262. CS+O=CO+S 1.00E+13 0.0 0.0 263. CO+S+M=COS+M 1.00E+13 0.0 0.0 264. CH+SO=CO+SH 1.00E+13 0.0 0.0
 Previous |  Table of Contents |  Next |