 exp(0.009 T)) (Gupta (1992)) ; other important factors are residence time and oxygen concentration, and they are significant insofar as they affect flame temperature.
exp(0.009 T)) (Gupta (1992)) ; other important factors are residence time and oxygen concentration, and they are significant insofar as they affect flame temperature.
The amounts of NO emitted from larger systems depend on the combustion hardware used (Sawyer and Starkman (1968)) . Considering NO emissions by Emission Index (EI, milligrams of NO per gram of fuel), spark-ignition engines yield EI = 16.3 whereas regenerative gas turbines reach EI = 13.5 and aircraft turbojets have EI = 5.5.
Other practical factors have also been studied (Cunningham (1978)) : When burning residual fuel oils in boilers air preheat has considerable influence on NO formation, whereas oil preheat showed little influence on NO. Conditions that favour high combustion intensities yield relatively higher NO emissions (Nimmo et al. (1991)) , such as small droplet size (Sarv et al. (1983)) , narrow spray angle, etc.
The NOX concentration in the exhaust of an oil-fired boiler (Gills (1973)) indicates that the NOX concentration decreases with excess air. Also the boiler size plays an important role on the concentration of NOX in the flue gases. Factors like the method of firing have little influence.
Maximum formation of NO occurs in gas turbines when the temperature is at its peak and at an equivalence ratio between 0.8 and 1.0 (Sawyer and Starkman (1968)) . The most important factor affecting NO formation in gas turbines is flame temperature (NOX  exp(0.009 T)) (Gupta (1992)) ; other important factors are residence time and oxygen concentration, and they are significant insofar as they affect flame temperature.
exp(0.009 T)) (Gupta (1992)) ; other important factors are residence time and oxygen concentration, and they are significant insofar as they affect flame temperature.
Modelling of NO formation by Bartok et al. shows the effect of several variables (Bartok et al. (1971)) :
O2  O + O O + O
| reac 1 |
O + N2  NO + N NO + N
| reac 2 |
N + O2  NO + O NO + O
| reac 3 |
N + OH NO + H NO + H
| reac 4 |
which, together with reactions 1, 2 and 3, is known as the extended Zeldovich mechanism.
The formation of NO via the Zeldovich mechanism is controlled by reaction 2 due to its very high activation energy (E = 314 kJ/mol). For this reason thermal-NOX shows a strong exponential dependence on temperature. The contribution of thermal-NO to the total NO formation is small below 1,370 °C (Gupta (1992)) , but becomes very important above 1,400 °C. Thus the peak flame or combustion temperatures are used as an indication of the importance of thermal-NO.
Other factors which also affect NOX formation are fuel/air mixing processes (related to local levels of excess air), combustion intensity and pre-heating of the combustion air. Thermal-NO has also been shown to increase linearly with residence time.
Several mathematical expressions have been derived to estimate the rate of thermal-NO formation in combustion systems.
If the processes leading to the formation of thermal-NOX occurred long after those of combustion, the amounts of NO could be calculated from the equilibrium conditions (Bowman (1979)) . Thus assuming a steady-state approximation for the N atom concentration, the formation rate of NO would involve the knowledge of the local temperature and the concentrations of O2, N2 and OH only, these being obtained from the post-combustion conditions. The following expression shows the large dependence of NO formation on temperature and oxygen concentration:
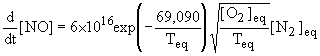
Toof (Toof (1986)) found the residence time for NO formation in gas turbines to be proportional to the velocity of the air jet entering the combustor (V), the diameter of the combustor (D), and also to a function of the amount of excess air (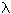 ):
):
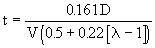
Another attempt to estimate the formation of NO in gas turbines was given by Sawyer and Starkman (Sawyer and Starkman (1968)) . Based only on the reaction:
O+ N2  NO + N NO + N
| reac 2 |
where the O atom concentration is fixed by the equilibrium
O2  O + O O + O
| reac 1 |
and assuming that the level of NO is fixed by the kinetics of formation rather than from equilibrium formation in the primary zone, they proposed the following expression:
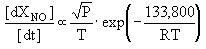
It shows a very strong dependence on temperature and inverse dependence on pressure, which are consistent with experimental results. The reverse reactions which would result in conversion of NO back into N2 and O2 are relatively slow in gas turbines (Starkman et al. (1971)) . Thus, NO, once formed, does not decrease in concentration at an appreciable rate by cooling.
This anomalous concentration of NO is said to be due to a different route from that to thermal-NO. Such rapidly-formed NO (produced before thermal-NO, and thus always within a given residence time) is named "prompt-NO".
Various arguments have been proposed to explain the rapid formation of these anomalous quantities of NO. Some authors postulate that in lean and near-stoichiometric flames they are caused by an overshoot of radical concentrations (O, OH) above the equilibrium values, which in turn has the effect of enhancing thermal-NO. A simple approach to the radical species involved is given by the pool of radicals formed in the following set of reactions (Burgess and Langley (1991)) , which are locally equilibrated:
O + H2  OH + H OH + H
| reac 5 |
H + O2  OH + O OH + O
| reac 6 |
H2 + OH  H + H2O H + H2O
| reac 7 |
O and OH radicals would thereafter enter the mechanism of thermal-NO formation. Accurate values of the temperature and radical concentrations used in calculations of the thermal-NO mechanism yielded results that were closer to experimental values. N2O has been suggested to play an important role in the low temperature (<1,225 °C) combustion of lean CO-air mixtures, as radical concentration overshoots cannot explain the increase of NO formation (Bowman (1979)) . The following mechanism was proposed:
N2 + O + M  N2O + M N2O + M
| reac 8 |
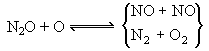
| reac 9 |
N2O + H  N2 + OH N2 + OH
| reac 10 |
However, kinetic modelling demonstrates that N2O does not play a significant role at high temperatures, and such are the conditions in internal combustion engines and other combustion hardware.
Finally, although [O] and [OH] were found to be high in fuel-rich mixtures, they do not account for the large formation of NO. A more plausible explanation is provided by reactions involving hydrocarbon fractions and atmospheric nitrogen as the source of nitrogen-containing radicals, which are eventually oxidised to form NO. The following reactions have been proposed by several authors (Bowman (1979), Glassman (1986), Toof (1986), Williams (1979)) :
Fuel, RH  CH, C2 CH, C2
| reac 11 |
C + N2  CN + N CN + N
| reac 12 |
C2 + N2  2 CN 2 CN
| reac 13 |
CH + N2  CN + HN CN + HN
| reac 14 |
CH + N2  CNH + N CNH + N
| reac 15 |
CH2 + N2  HCN + N HCN + N
| reac 16 |
C2H + N2  HCN + CN HCN + CN
| reac 17 |
HCN + oxidant  NHi NHi
| reac 18 |
In a further stage, NHi species yield NO by:
NH + H  N + H2 N + H2
| reac 19 |
N + O2  NO + O NO + O
| reac 3 |
N + OH  NO + H NO + H
| reac 4 |
in a similar system of reactions to that of fuel-NO (see next section).
The formation of HCN has been observed experimentally in all hydrocarbon-rich flames. When the equivalence ratio is below 1.4 there are enough oxygen radicals to react with HCN in order to form amine compounds and eventually NO.
Fenimore (Fenimore (1971)) estimated that at stoichiometric equivalence ratio in the turbulent flame of combustors, prompt-NO was responsible for 30 % of the total NOX emissions from gas turbines, according to:
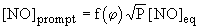
where:
f( ) : an empirical function of the equivalence ratio, whose value is 0.016 for stoichiometric mixtures (
) : an empirical function of the equivalence ratio, whose value is 0.016 for stoichiometric mixtures ( = 1).
= 1).
P: pressure, atm
[NO]eq: equilibrium concentration of NO
The previous formula reveals that prompt-NO is highly dependent on pressure. Experimental results on premixed ethylene/air flames at pressures up to 18 atm have confirmed this finding (Gupta (1992)) . However, unlike thermal-NO, its dependence on temperature is very weak. At temperatures above 1,325 °C prompt-NO constitutes only a small fraction of total-NO, if compared to thermal-NO.
HCN + O  NCO + H NCO + H
| reac 20 |
NCO + H  NH + CO NH + CO
| reac 21 |
whereas in fuel-rich systems the mechanism is through NH2:
HCN + OH  HNCO + H HNCO + H
| reac 22 |
HNCO + H  NH2 + CO NH2 + CO
| reac 23 |
NH2 + H  NH + H2 NH + H2
| reac 24 |
The subsequent oxidation of the amine species is a rapid process which occurs via two routes, depending on the availability of oxidant in the combustion environment (Toof (1986)) :
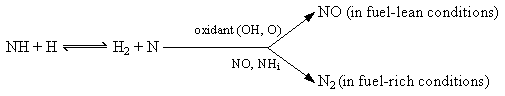
Once NO is formed it plays a synergistic role against amine oxidation by reduction to molecular nitrogen. As a result, the fraction of fuel-nitrogen converted into NO depends on the relative rates of the parallel paths
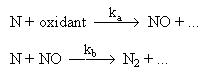
Once NO has been formed its disappearance can occur by conversion into HCN with CH and CH2 radicals. HCN can then re-enter the NO formation process.
The overall formation of fuel-NO has a weaker dependence on flame temperature than thermal-NO. However, its dependence on oxygen concentration (Gupta (1992)) is very strong: Relatively high yields of fuel-NO are attained in lean and stoichiometric mixtures, whereas low yields are found from fuel-rich mixtures (Bowman (1979), Pfefferle and Churchill (1986)) as in reducing atmospheres the formation of N2 is favoured with respect to that of NO. Also, the extent of the conversion is an inverse function of the fuel-N content (Fenimore (1972), Nimmo et al. (1995), Williams (1979)): For low levels of fuel-bound nitrogen (0.1 %), conversion may reach 100 %, whereas for higher yields (0.5 %) it is reduced to about 50 %.
The conversion of different forms of fuel-N into NO is the subject of controversy among researchers. Additives with low boiling point are reported to produce lower yield than those with high-boiling point (Gupta (1992)) . However, some other authors state that the conversion is nearly independent of the parent molecule (Nimmo et al. (1995), Pfefferle and Churchill (1986), Williams (1979)) . The speed at which intermediate species are formed seems to vary for different fuel-nitrogen compounds (Nimmo et al. (1995), Song et al. (1981)) , being faster for nitrogen compounds of low boiling point.
Attempts have been made to estimate the amounts of fuel-NO formed in combustion systems. Fenimore (Fenimore (1972)) derived an expression for the calculation of the conversion fraction of fuel-N into NO. By assuming that amine species always take part in the mechanism, the following formula was proposed for the addition of various nitrogen compounds to premixed ethylene-air flames, in which fuel-NO is formed prior to thermal-NO:
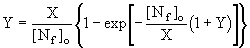
where:
Y: NO conversion fraction = 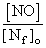
[Nf]o: Total N in the added nitrogen compound, ie the amount of NO which would be formed if all the fuel nitrogen was converted to NO
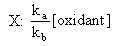
As opposed to other authors, Fenimore proposed that the oxidant involved in NHi transformation to NO was the OH radical.
Another correlation was provided by Soete (Bowman (1979)) :
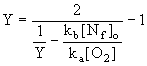
in which it was assumed that the N atom was the only nitrogen intermediate that reacts with oxidant or NO, and that the formation of both NO and N2 would be governed by the standard reactions of the Zeldovich mechanism:
 NO + O NO + O
reac 3 = a
| N + NO |  N2 + O N2 + O
reac -2 = b
| |
In lean flames with small amounts of fuel-N, ie ka[O2]>>kb[Nf]o the conversion fraction of NO would rise to unity, ie all fuel-nitrogen would be converted to NO.
 Previous |  Table of Contents |  Next |