 2 NO2 reac 25
2 NO2 reac 25At low temperature (below 1,100 °C) the reaction
 2 NO2 reac 25
2 NO2 reac 25is slow. However, other reactions are important at low temperatures below 900 °C, such as (Cernansky and Sawyer (1974)) :
HNO2 + O  NO2 + OH NO2 + OH
| reac 26 |
NO + O2  NO2 + O2 NO2 + O2
| reac 27 |
HNO2 + OH  NO2 + H2O NO2 + H2O
| reac 28 |
An important mechanisms proposed for NO2 formation and disappearance is the following (Glassman (1986)) :
NO + HO2  NO2 + OH NO2 + OH
| reac 29 |
NO2 + O  NO + O2 NO + O2
| reac 30 |
NO oxidises to NO2 very rapidly through reaction 29 in the early parts of the flame, where the concentration of HO2 is high. Subsequently NO2 may disappear in reaction 30 as a surge of O radicals is formed concurrently. Both reactions 29 and 30 are fast at low temperatures (Bowman (1979)) .
HO2 radicals abound in initial low-temperature, oxygen-rich flame zones, where they are formed via the reaction (Hori (1988)) :
 HO2 + N2 reac 31
HO2 + N2 reac 31The balance between its formation and destruction is mainly determined by temperature. Thus, for a 0.1 O2 mole fraction, 20 ppm HO2 are found at 1,125 °C, 50 ppm at 725 °C and 400 ppm at 230 °C. It can be seen that formation of NO2 via HO2 is inversely dependent on the temperature. Under normal circumstances NO2 only exists as a transient species in combustion systems. NO2 would only be emitted if some kind of quenching was applied on the re-conversion reactions of NO2 into NO. Rapid mixing of hot and cold regions can act so that reaction 30 is halted (Hori (1988)) . High NO2/NOX ratios have been found in cooler regions near the combustion zone (Bowman (1979)) . High equivalence ratios increase the NO2/NOX ratio to values close to unity partly due to an excess of HO2 radicals. In addition, OH radicals in fuel-rich flames can react with unburned hydrocarbon species in order to form alkyl peroxy radicals, which are very efficient at oxydising NO to NO2 (Hargreaves et al. (1981)) . They may also increase the amount of HO2 radicals available (Hori (1988)) .
Cernansky and Sawyer (Cernansky and Sawyer (1974)) found evidence for the formation of NO2 in the early, fuel-rich regions of flames. Although the stoichiometry of the mixture is fuel-rich, oxygen penetration can provide O concentrations required for the HO2 mechanism. The formation of nitrogen dioxide was again explained as a result of rapid cooling and turbulent mixing of the combustion gases, in situations where oxygen, low temperature and superequilibrium radical concentrations exist. Large superequilibrium concentrations of HO2 radicals are formed then, and they can outlive H, O and OH radicals.
Such explanations are not totally conclusive and other hypotheses are sustained. Some authors argue that the levels of NO2 reported in the flame zone are the consequence of reactions occurring due to rapid quenching of the combustion gases on the probe wall through reaction:
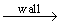 NO2 reac 32
NO2 reac 32or by means of high concentrations of HO2 formed at low temperature in the sampling system (Bowman (1979), Hargreaves et al. (1981)) . The same low-temperature oxygen concentration and superequilibrium radical concentrations that favour NO2 formation in the flame can be found in sampling probes (Cernansky and Sawyer (1974)) . Hargreaves et al. (Hargreaves et al. (1981)) also showed that sampling pressure can alter the NO2/NO ratio substantially.
The removal of NO2 is promoted by large concentrations of radicals present at high temperatures (Bowman (1979)) in reactions such as:
NO2 + H  NO + OH NO + OH
| reac 33 |
NO2 + O  NO + O2 NO + O2
| reac 30 |
 Previous |  Table of Contents |  Next |