 = 0.833)
= 0.833) = 0.833)
= 0.833)
 = 0.833
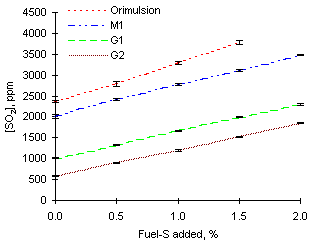
= 0.833As expected, the measured concentrations of SO2 increase with the injection of SO2-gas to the combustion system. Concentrations of SO2 rose by an average 472 ppm with every 0.5 % fuel-S increase to Orimulsion. The increases were lower with the heavy gas oils investigated, between 316 and 363 ppm for each 0.5 % fuel-S increase, due to smaller additions of SO2 being required to meet the percentage of fuel-S.
However, in all cases the increases of SO2 concentrations were greater than the values estimated prior to experiments. Calculations showed that injection of 0.5 % fuel-S as SO2 should cause an increase of 418 ppm from Orimulsion, whereas only 296 ppm are to be expected from heavy gas oils in fuel-lean conditions.
 = 1.000)
= 1.000)
 = 1.000
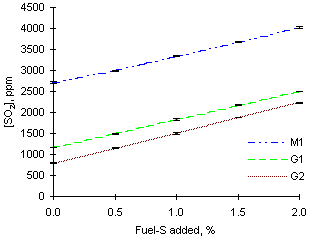
= 1.000For the same reason, it is foreseeable that addition of identical masses of SO2 dopant would cause higher increases of the concentration of sulphur dioxide.
Calculations showed that the measured concentration of SO2 should rise by 350 ppm with an increase of 0.5 % S dopant. However, the increases of SO2 concentration measured on addition of sulphur dioxide were similar to those obtained in fuel-lean conditions and lower than calculated ones in most cases. Values of SO2 emissions increased by an average 340 ppm for each 0.5 % fuel-S equivalent added.
 = 1.200)
= 1.200)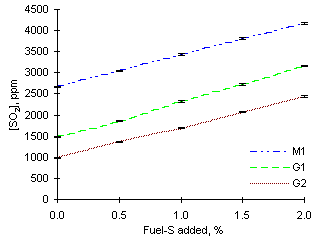
 = 1.200
= 1.200The concentrations of SO2 measured in fuel-rich conditions were highest among all equivalence ratios considered.
However, increases of SO2 emissions with increasing amounts of dopant, although slightly higher, remained similar to those in fuel-lean and stoichiometric conditions. The average increase of SO2 emissions per 0.5 % fuel-S increase was 384 ppm, whereas calculations indicate that these should be increased by 414 ppm.
The disparity between these figures denotes that SO2 may be reacting after injection into the combustion chamber. A possible explanation could be the reduction of SO2 to other sulphurous species, which was discussed in chapter III. Once again, this is substantiated by yellow solid deposits found in the exhaust system of the drop-tube furnace. Also, not all sulphur contained in the fuel is transformed into SO2 during combustion in fuel-rich conditions. As shown in chapter III, the conversion of fuel-S into SO2 decreases with the increase of the equivalence ratio.
 Previous |  Table of Contents |  Next |