 = 1.200)
= 1.200) = 1.200)
= 1.200)
As in stoichiometric mixtures, the peak of NO formation at  = 1.200 occurs before the maximum flame temperature, between 200 and 250 mm, with a subsequent decrease to the concentrations of NO that are eventually emitted.
= 1.200 occurs before the maximum flame temperature, between 200 and 250 mm, with a subsequent decrease to the concentrations of NO that are eventually emitted.
The main nitrogenous compound emitted under fuel-rich conditions is NO2. At 900 °C furnace wall temperature and between 250 and 400 mm distance from the atomiser, NO2 is formed at a steady rate of 0.33 ppm-wet/mm for fuel M1 and 0.24 ppm-wet/mm for fuel G1. This can be observed in Figures 46 and 47. However, for the large amounts of NO2 formed at that equivalence ratio no similar transient amounts of NO are detected. Which is the mechanism for NO2 formation in these conditions? Is NO2 being formed through NO + HO2 in a very fast reaction or via another mechanism such as reaction with hydrocarbon chains? In principle, it seems unlikely that NO2 would be formed by means of the HO2 reaction as the flame temperature is excessively high. In addition, the steady rates of formation observed suggest a mechanism that is not dependent on the flame temperature (the value of the rate constant for reaction 29, NO + HO2  NO2 + OH given by Glaenzer and Troe (Miller and Bowman (1989)) for the relevant temperature range is constant and independent of temperature - see reaction 189 in "Appendix III" for a value of the reaction rate constant). Also, the conversion of fuel-N into NO2 does not seem to depend on the fuel-N content at
NO2 + OH given by Glaenzer and Troe (Miller and Bowman (1989)) for the relevant temperature range is constant and independent of temperature - see reaction 189 in "Appendix III" for a value of the reaction rate constant). Also, the conversion of fuel-N into NO2 does not seem to depend on the fuel-N content at  = 1.200. A similar effect relating the fuel-N content and the equivalence ratio was observed by Pfefferle and Churchill (Pfefferle and Churchill (1986)) from ethane flames doped with various amounts of ammonia: Different dopant concentrations yielded comparable NOX emissions from fuel-rich flames.
= 1.200. A similar effect relating the fuel-N content and the equivalence ratio was observed by Pfefferle and Churchill (Pfefferle and Churchill (1986)) from ethane flames doped with various amounts of ammonia: Different dopant concentrations yielded comparable NOX emissions from fuel-rich flames.
High emissions of NO2 in fuel-rich systems have been observed by other researchers. Courtemanche and Levendis (Courtemanche and Levendis (1997)) found the ratio of [NO2/NO] emissions to increase when solid fuels were burned at fuel-rich conditions. Hori (Hori (1988)) also found very high [NO2/NOX] ratios in fuel-rich, air-cooled propane-air flames. The ratio was increased by two factors: The mixing of hot combustion gases with cold air and the sampling of gases with a water-cooled probe. NO2 was presumed to form in the water-cooled sampling probe due to the rapid but steady cooling of the combustion gases, which causes the formation of HO2 radicals. A similar explanation was given by Malte and Kramlich (Malte and Kramlich (1980)) .
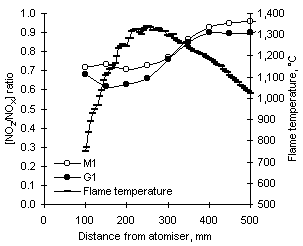
 = 1.200 and 900 °C
= 1.200 and 900 °C
A graph showing the axial profile of the [NO2/NOX] ratio and the flame temperature profile from experiments performed with fuels M1 and G1 at  = 1.200 and 900 °C furnace wall temperature is shown in Figure 97. The profiles of [NO2/NOX] ratio show similarities with those reported by Hori with a water-cooled probe at
= 1.200 and 900 °C furnace wall temperature is shown in Figure 97. The profiles of [NO2/NOX] ratio show similarities with those reported by Hori with a water-cooled probe at  = 1.510 (gas sampling from the drop-tube furnace was also performed with a water-cooled probe). However, NO2 is formed in the drop-tube furnace at a constant rate throughout the range of flame temperatures encountered from 150 to 500 mm, which includes zones of increasing, maximum and decreasing temperatures (see Figure 97). Constant formation of NO2 is not consistent with a pattern of changing temperature.
= 1.510 (gas sampling from the drop-tube furnace was also performed with a water-cooled probe). However, NO2 is formed in the drop-tube furnace at a constant rate throughout the range of flame temperatures encountered from 150 to 500 mm, which includes zones of increasing, maximum and decreasing temperatures (see Figure 97). Constant formation of NO2 is not consistent with a pattern of changing temperature.
An alternative hypothesis can be formulated. Irrespective of the axial flame temperature profile, the furnace walls are at a constant temperature of 900 °C. Combustion gases near the furnace walls will be at a temperature which is more favourable for the formation of HO2 and, consequently, NO2. Also, oxygen needed to form HO2 radicals is still available (albeit in low concentrations) up to 500 mm from the atomiser. NO2 thus formed can subsequently diffuse back into the furnace central axis, giving place to the concentrations measured. Given the inverse relationship of HO2 radicals with temperature, lower concentrations of NO2 would be expected at higher furnace wall temperatures. However, the exhaust concentrations of NO2 increased from 900 to 1,100 °C for fuel M1, whereas a very minor decrease was obtained from fuel G1.
Another likely source of high NO2 concentrations is the reaction of NO with unburned hydrocarbons, present in fuel-rich systems such as that in the drop-tube furnace at  = 1.200. This possibility was contemplated by Jaasma and Borman (Jaasma and Borman (1980)) to account for increased NO2 levels in diffusion flames with hydrocarbon-doped combustion air. Numerical calculations were performed by Sano (Sano (1985)) which yielded increased concentrations of NO2 when a cool stream of air containing CH4 was added to combustion gases containing nitric oxide. Methane is assumed to enhance the formation of H radicals which, in turn, would increase HO2 concentrations. Bromly et al. (Bromly et al. (1988)) also found that concentrations of hydrocarbons could oxidise NO to NO2 at relatively high temperatures. This effect was augmented by the presence of CO and H2O. The mechanism proceeds through the formation of hydroperoxyl radicals and other peroxides that can oxidise NO to NO2. Later, Hori et al. (Hori et al. (1992)) found that propane was most effective at promoting the oxidation of NO into NO2. Also, it was favoured at relatively high temperatures where the formation of active species was sufficiently high.
= 1.200. This possibility was contemplated by Jaasma and Borman (Jaasma and Borman (1980)) to account for increased NO2 levels in diffusion flames with hydrocarbon-doped combustion air. Numerical calculations were performed by Sano (Sano (1985)) which yielded increased concentrations of NO2 when a cool stream of air containing CH4 was added to combustion gases containing nitric oxide. Methane is assumed to enhance the formation of H radicals which, in turn, would increase HO2 concentrations. Bromly et al. (Bromly et al. (1988)) also found that concentrations of hydrocarbons could oxidise NO to NO2 at relatively high temperatures. This effect was augmented by the presence of CO and H2O. The mechanism proceeds through the formation of hydroperoxyl radicals and other peroxides that can oxidise NO to NO2. Later, Hori et al. (Hori et al. (1992)) found that propane was most effective at promoting the oxidation of NO into NO2. Also, it was favoured at relatively high temperatures where the formation of active species was sufficiently high.
The numerical model used in the present thesis shows that the concentrations of hydrocarbon fractions increased in fuel-rich mixtures ( = 1.200) with respect to those under stoichiometric conditions (
= 1.200) with respect to those under stoichiometric conditions ( = 1.000). This could lead to greater concentrations of NO2 via the hydroperoxyl mechanism.
= 1.000). This could lead to greater concentrations of NO2 via the hydroperoxyl mechanism.
At fuel-rich equivalence ratios the formation of NO2 shows very little sensitivity to the furnace wall temperature. It is predictable that increasing furnace wall temperatures will increase the flame temperature. Since NO2 represents the largest proportion of nitrogenous species formed, the concentrations of NOX are determined by those of NO2.
 Previous |  Table of Contents |  Next |