 = 1.000)
= 1.000) = 1.000)
= 1.000)
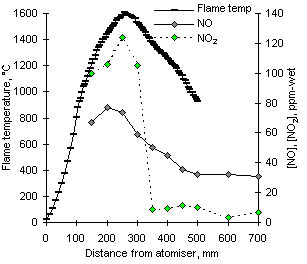
A large drop of NO concentrations was observed to occur from fuel-lean to stoichiometric conditions. At  = 1.000 the formation of NO peaks before the maximum flame temperature, between 200 and 250 mm, followed by a decrease which allows certain, although low, amounts of NO to be eventually emitted (see Figure 96).
= 1.000 the formation of NO peaks before the maximum flame temperature, between 200 and 250 mm, followed by a decrease which allows certain, although low, amounts of NO to be eventually emitted (see Figure 96).
 = 1.000 and 900 °C
= 1.000 and 900 °C
Experimental results show that at  = 1.000 nitric oxide is not transformed into NO2, as the concentrations of NO2 also decrease sharply after reaching a maximum value at 250 mm, coincidental with the maximum flame temperature. NO formed in stoichiometric conditions is likely to be converted to HCN again by means of reactions with CH and CH2 radicals, which may be present. Another alternative mechanism is the reduction of NO to form N2 with amine species (Pfefferle and Churchill (1986)) , which is postulated as part of the fuel-NO mechanism in high concentrations of nitric oxide:
= 1.000 nitric oxide is not transformed into NO2, as the concentrations of NO2 also decrease sharply after reaching a maximum value at 250 mm, coincidental with the maximum flame temperature. NO formed in stoichiometric conditions is likely to be converted to HCN again by means of reactions with CH and CH2 radicals, which may be present. Another alternative mechanism is the reduction of NO to form N2 with amine species (Pfefferle and Churchill (1986)) , which is postulated as part of the fuel-NO mechanism in high concentrations of nitric oxide:
NH2 + NO  N2 + H2O N2 + H2O
| reac 39 |
NH + NO  N2 + OH N2 + OH
| reac 87 |
N + NO  N2 + O N2 + O
| reac -2 |
The concentrations of NO2 measured at short distances are larger than in fuel-lean conditions, possibly due to the greater influence of the RO2 mechanism (see next paragraph) as the equivalence ratio increases.
The composite effect of low emissions of both NO and NO2 is the low conversion of fuel-N to NOX at  = 1.000.
= 1.000.
 Previous |  Table of Contents |  Next |