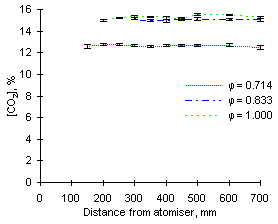
 = 0.833. The readings yield values approaching 16 % CO2 for both fuels investigated.
= 0.833. The readings yield values approaching 16 % CO2 for both fuels investigated.
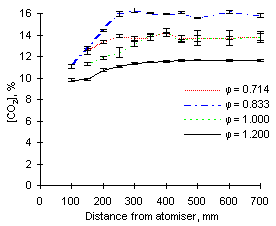
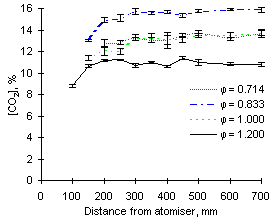
The most outstanding feature observed from the CO2 signals recorded is that the highest concentrations of carbon dioxide are recorded at  = 0.833. The readings yield values approaching 16 % CO2 for both fuels investigated.
= 0.833. The readings yield values approaching 16 % CO2 for both fuels investigated.
Both fuels produce similar amounts of CO2 at exhaust when burned at  = 0.714 and 1.000, ie approximately 13.8 %.
= 0.714 and 1.000, ie approximately 13.8 %.
Lastly, minimum formation and emission of CO2 was detected at fuel-rich conditions ( = 1.200), with concentrations of CO2 at about 11 % (see Figures 76 and 77).
= 1.200), with concentrations of CO2 at about 11 % (see Figures 76 and 77).
Carbon dioxide is formed rapidly in the first 100 mm of the furnace. Subsequently, its concentration increases to reach values close to those of emission at approximately 250 mm.
| 
|
| Figure 76: CO2 concentrations from fuel M1 at 900 °C | Figure 77: CO2 concentrations from fuel G1 at 900 °C |
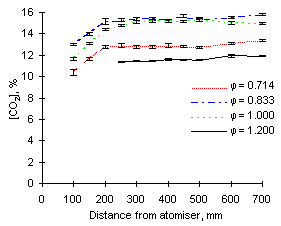
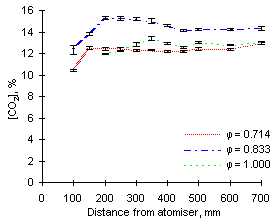
The exhaust concentrations of carbon dioxide obtained at 1,100 °C were essentially similar to those at 900 °C for both fuels investigated. However, at  = 1.000 fuel M1 produced a slightly higher amount of CO2 than fuel G1 (15.0 vs 14.3 %).
= 1.000 fuel M1 produced a slightly higher amount of CO2 than fuel G1 (15.0 vs 14.3 %).
| 
|
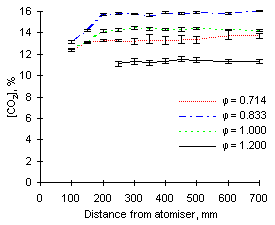
| Figure 78: CO2 concentrations from fuel M1 at 1,100 °C | Figure 79: CO2 concentrations from fuel G1 at 1,100 °C |
A major difference is, however, given by the fact that such concentrations are reached at shorter distances, ie earlier residence times. At 1,100 °C, formation of CO2 reaches completion at about 200 mm from the atomiser, whereas it is achieved at 250 or 300 mm at 900 °C.
| 
|
| Figure 80: CO2 concentrations from fuel M1 at 1,200 °C | Figure 81: CO2 concentrations from fuel G1 at 1,200 °C |
No substantial changes in the emissions of CO2 were observed as the furnace wall temperature was increased to 1,200 °C.
Again, the emissions of CO2 at stoichiometric conditions were higher for fuel M1 than fuel G1 (15.3 vs 13.0 %).
 Previous |  Table of Contents |  Next |