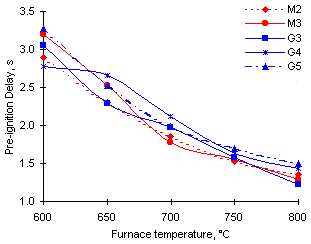
Figure 25: Calculated Pre-ignition Delay for a standard 1 mm initial diameter droplet

The trend observed from the experimental results is that of an overall decrease of ti throughout the range of temperatures studied (see Figure 25). At high combustion temperatures the results converge towards a narrow range of values. Results previously obtained by Malik and Burgess (Malik and Burgess (1985)) also showed the Pre-ignition Delays of different fuels to become coincidental as the ambient temperature rose.
The Pre-ignition Delay can be considered the result of three distinct successive processes. For each of these processes a time delay can be defined (Malik (1986)) :
The values of the Pre-ignition Delay measured are lower than those obtained with other heavy fuels, such as residual oils and coal-water slurries in the same experimental facility (Burgess and Ghaffari (1989), Malik and Burgess (1985)) . This can be attributed to the presence of lighter fractions of low volatility that form an ignitable cloud around the droplet more quickly.
Although no major differences were observed in the Pre-ignition Delay of the fuels studied, the experimental results reveal three distinct behaviour patterns:
Group 2 exhibits the longest Pre-ignition Delay among all samples studied. Group 1 shows intermediate values whilst Group 3 (fuel G3) becomes the most easily ignitable oil sample at 800 °C, with the shortest Pre-ignition Delay.
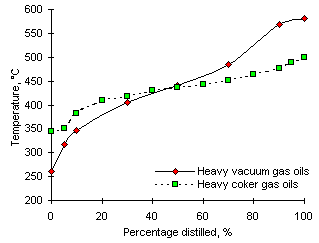
The fact that the pre-ignition delays are longer for some heavy vacuum gas oils than for the heavy coker gas oils does not agree with the characteristics of their distillation ranges. These show that the evaporation of components from the heavy coker gas oils commences at higher temperatures than those in the heavy vacuum gas oils (see Figure 26). The significance of this fact should be the faster production of volatiles from heavy vacuum gas oils.
However, differences in the chemical composition of both types of fuel can establish the conditions for different Pre-ignition Delays. With similar contributions from the heating-up delay (th) and the evaporation delay (te) at the same experimental conditions, differences in the chemical delay (tchem) can determine the varying values of ti. These differences can be caused by the chemical composition of both types of fuels. One example is the aromatics content, 33 % in heavy coker gas oils vs 23 % in heavy vacuum gas oils, as detailed in "Appendix II". The flammability of (fuel:air) mixtures is greatly dependent on the chemical composition of the fuel and its vaporised components. Shorter Pre-ignition Delays and lower Ignition Temperatures are obtained from the for heavy coker gas oils, which is also explained in section "4.5. Ignition Temperature (Ti)" as flammability is reached in a shorter period of time.
The influence of the furnace temperature on the Pre-ignition Delay can be expressed by the following Arrhenius-type equation (Malik (1986)) :
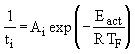
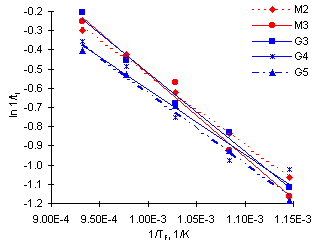
Figure 27 shows a plot of ln 1/ti vs 1/TF. The values of the activation energy and the pre-exponential factor obtained are shown in the following Table:
| Fuel | Eact, kJ/mol | Ai, 1/s | r2 |
|---|---|---|---|
| M2 | 30.3 | 22.5 | 0.997 |
| M3 | 36.1 | 45.2 | 0.986 |
| G3 | 34.0 | 35.5 | 0.989 |
| G4 | 28.1 | 16.0 | 0.946 |
| G5 | 30.6 | 21.3 | 0.992 |
As explained earlier, the overall activation energy is the sum of the activation energies of the processes that precede ignition, that is, heating-up, chemical and evaporation processes (Malik (1986)) . According to the results shown in Table 4, fuel G4 would be, in principle, the most ignitable fuel studied. However, similar slopes show that no large differences exist among the calculated activation energies, and they would possess similar ignition characteristics. High activation energies are normally associated with high aromatic contents. Unfortunately, no detailed information on the aromatic content of the fuels was available.
 Previous |  Table of Contents |  Next |