3. Definitions
This is the period of time elapsing from the arrival of the droplet suspended on the thermocouple at the centre of the furnace until the homogeneous ignition of the vapour surrounding the sample. The processes taking place during this stage involve mainly heating-up of the fuel, evaporation of volatile components, cracking of fuel components and gas phase reactions that eventually lead to the ignition of the fuel.
The Pre-ignition Delay relates to the initial droplet diameter in the following way:
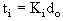
The Flame Time comprises the period between the onset of homogeneous ignition around the droplet and its extinction. During the flame stage cracking and further volatilisation of material from the droplet occur.
The flame resulting from ignition is detected by means of a planar photodiode. The duration of the Flame Time is obtained from the photodiode trace on the oscilloscope screen.
The Flame Time is related to the squared power of the initial droplet diameter:
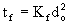
This parameter includes the time taken by all measurable combustion processes taking place in the sample droplet. In the case of the samples studied it includes the Pre-ignition Delay and the Flame Time, and it is calculated by their addition in every single experiment (t = ti + tf). The coke burn-out time (tc), ie, the time taken by any solid carbon residue (if present) to disappear, was not included in the evaluation of the Total Combustion Time in these experiments (see section "3.6. Peak temperature").
The Total Combustion Time is related to the squared power of the initial droplet diameter:
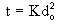
The Ignition Temperature is the temperature recorded by the combustion thermocouple at the onset of homogeneous ignition. The temperature measured is that of the centre in the droplet.
The Ignition Temperature was obtained from hard records of the combustion sequences.
According to Taylor and Burgess (Taylor and Burgess (1988)) the Ignition Temperature is related to the inverse of the initial droplet diameter by:
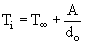
where T represents the theoretical ignition temperature of a large pool of oil, ie at do =
represents the theoretical ignition temperature of a large pool of oil, ie at do =  .
.
It is the minimum droplet diameter of a given fuel which exhibits homogeneous ignition at certain experimental conditions of furnace temperature. Ignition is understood as the presence of a flame emerging from the droplet surroundings.
Ignition takes place when the mixture of gases around the droplet is within the ignition limits at the furnace temperature. Small droplets have to produce vapour at a sufficiently fast rate so that the right mixture is formed. At low furnace temperatures the fuel diffuses away before the mixture is formed, and thus ignition is not reached. At higher temperatures the ignitable mixture is attained more rapidly due to increased volatilisation.
Since only a limited number of runs was performed with each fuel, the determination of the Critical Diameter for Ignition was imprecise. Experimentally the Critical Diameter for Ignition is assumed to lie between the largest initial droplet diameter showing lack of ignition and the lowest diameter showing homogeneous ignition.
The Peak Temperature is the highest temperature reached in the centre of the burning sample. The existence of a peak reveals the presence of further combustion reactions taking place after the flame is quenched. Such reactions are usually heterogeneous processes caused by the diffusion of oxygen towards a coke particulate formed from the heavy components of the fuel. The measurement of the Peak Temperature is made from hard records of the combustion sequences.
Pollutant formation and interaction in the combustion of heavy liquid fuels
Luis Javier Molero de Blas, PhD thesis, University of London, 1998
© Luis Javier Molero de Blas
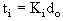
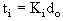
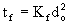
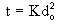
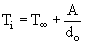
 represents the theoretical ignition temperature of a large pool of oil, ie at do =
represents the theoretical ignition temperature of a large pool of oil, ie at do =  .
.
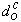 )
)

