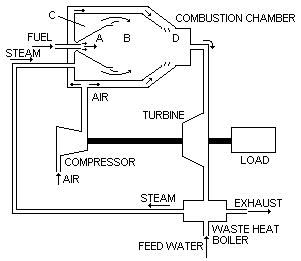
Figure 15: Gas turbine cycle with steam generator and heat recovery as in Patent Application GB 2,187,273
The efficiency of a gas turbine cycle that uses ambient air as a working fluid can be improved by either increasing the turbine inlet temperature, or by introducing means to recover some of the energy from the turbine exhaust gases.
The turbine inlet temperature is governed by the temperature limits of the highly stressed turbine blades. Stoichiometric combustion temperatures for typical fuels are in the range of 1,870 to 2,200 °C, whilst turbine materials are limited to 760 to 980 °C.
The work output from a gas turbine depends upon the value of the exhaust temperature at the completion of expansion, which in turn depends upon the value of the specific heat ratio §. Greater temperature drop is obtained from fluids with low values of §. Performance can be greatly improved by expanding a mixture of air and steam. When the steam is generated by heat from the turbine exhaust gases, the cycle becomes thermally more efficient.
Inferior grade and relatively inexpensive petroleum fuels include petroleum fractions which boil at about 425 °C, compounds of sodium, calcium, nickel, iron and from 2 to 400 ppm of vanadium. They also have sulphur concentrations in the range from about 0.5 to 5 % by weight. The combustion products of these fuels are excessively corrosive and have a high capacity for ash formation. If a fuel of this type is burnt with near stoichiometric quantities of air, ie, 0 to 3 % excess air, the corrosion and fouling effects are substantially decreased. However, if larger quantities are used, ie, 5 to 500 % above stoichiometric, then ash is produced together with the combustion products and they form corrosive deposits. In conventional gas turbine operation the following pollutants are produced: NOX, SOX, CO and ash (compounds of Na, V, Ca, Mg, Fe, Ni, Si).
It is known that injection of water reduces the maximum temperature reached in the turbine cycle. However, solid particles in the water may have an adverse effect on the turbine parts.
This patent application presents a gas turbine cycle (Figure 15) in which the turbine exhaust gases are directed into a waste-heat boiler in order to raise saturated or supersaturated steam. High pressure steam injected adds to the mass of working fluid, leading to increased power and thermal efficiency.

However, in view of the presence of sulphur compounds within the fuel oil, the temperature reduction of the exhaust gas must be limited to avoid condensation. The gas within the pre-heater section should be kept above its acid dewpoint (see section "4.2.2.c. Sulphur trioxide"). A limiting value of about 150 °C has been used.
The final exhaust gases, containing a mixture of combustion products, air and steam are expelled into the atmosphere at atmospheric pressure and about 150 °C. The exhaust emissions have very low environmental pollution levels.
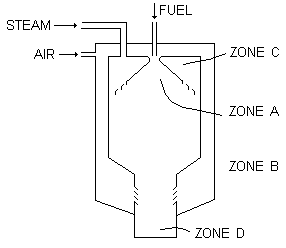
In the process within the combustor the formation of harmful compounds is restricted by limiting the amount of primary air (at a pressure of approximately 10 atmospheres) in zone A (see Figure 16) to be no significantly less than 3 % above stoichiometric and by completely surrounding this near adiabatic flame by a continuous curtain of steam. The atmosphere outside the flame but contained within zone A is completely free from air. The steam used is at a much lower temperature than that of the combustion products. Its amount is regulated to ensure that the temperature of the mixture of steam and combustion products is much less than the minimum temperature required to produce the sodium-vanadium products. Additional steam is introduced into zone B so that intimate contact takes place at the adiabatic flame boundaries. The internal cylindrical walls are protected from the effects of the high temperature. The amount of steam introduced into zone B may vary and the amount selected is that which achieves the balance between the approach temperature and the exit temperature from the waste heat boiler.
It should be noted that the exhaust gases contain large proportions of unburned oxygen and no attempt is made to combust it by auxiliary firing. Thus the basic concept of the present invention is to burn typical distillate fuels.
Although at the extremely low steam pressures encountered in this cycle (10.5 kg/cm2) the required quality of feed water treatment is low, some degree of treatment will be necessary to operate the waste heat boiler to prevent the possibility of the carry-over of impurities into the turbine section. The turbine can be started in a dry mode and if required can be operated continuously in the dry mode, because it will require 15-30 minutes from lighting the burner before steam can be raised and thus injected into the combustion chamber.
In the present arrangement an approximately stoichiometric mixture of fuel and air are introduced into the primary zone I (see Figure 17) chamber at about 6 atm. Combustion is almost complete in this section.
Simultaneously, steam is introduced into the annular area around the primary zone. Steam cools the casing walls of this and the secondary zone II. the steam is eventually introduced into the secondary zone II, where it mixes with the hot combustion gases. The amount of steam introduced into the secondary zone II is about 6 to 10 kilograms of steam per kilogram of fuel at a pressure of 5 to 13.3 atm.
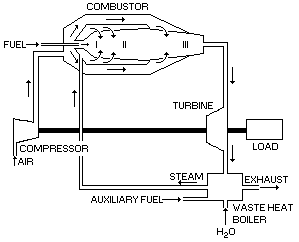
Finally, the exhaust gases are discharged to the turbine section. The temperature of the gases is between 1,350 and 1,700 °C, and its pressure is in the range of 3 to 13.3 atm.
The spent turbine gases are passed into a waste heat boiler, where they are utilised to raise steam at a pressure higher than that of the turbine cycle. Additional steam may be generated by an oil-fired boiler, which may be separated from the waste heat boiler.
The turbine is operated with essentially the stoichiometric amount of air (0 to 3 % excess), whereas in conventional turbines up to 500 % excess air is used. This is accomplished by the addition of steam. This low excess of stoichiometric air maintains the combustion products of the metals and sulphur in a low valence state so that the harmful deposits characteristic of the higher valence states are avoided. If quenching had been performed by air, the ash and sulphur would be oxidised, thus losing the benefits of the stoichiometric combustion.
 Previous |  Table of Contents |  Next |