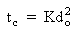
Criticism of the Suspended Droplet technique stems from various facts:
A variety of fuels have been studied in the past, ranging from coal slurries to heavy or distillate oils, lubricating oils, etc.
One of the earliest studies with the single suspended droplet technique was published by Masdin and Essenhigh (Masdin and Essenhigh (1958)). Droplets suspended from a silica fibre were subjected to both convective and radiative fields of variable intensity provided by two coils. By using pitch creosote as a sample fuel they observed that a fixed gas temperature was necessary for the droplets to ignite. Below such temperatures droplets would only evaporate.
In their experiments they observed the combustion of pitch creosote to undergo two stages:
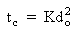
where K represents a burning constant, calculated from the experimental values of do and tc obtained from ciné records.
If the heat was intense enough the droplets would ignite. Cenospheres formed from droplets which underwent ignition, for which a convective field was needed, were half the size of those formed after simple evaporation in radiation field.
Similar equipment was used later by the same investigators (Masdin and Thring (1962)). They observed the changing character of the droplet size throughout the combustion of heavy residual oil. Swelling and contraction alternated, eventually to produce a solid residue (cenosphere) which combusted heterogeneously after the volatiles burned in a flame. They established that, although the process of burning is not uniform, a certain relationship exists between do (initial drop diameter) and tv (burning time of volatiles). The values of the proportionality constant K for heavy residual oils reported by the researchers were close to those obtained for kerosene, meaning that under equal conditions it takes similar time to burn the volatiles produced by any type of fuel.
Natarajan (Natarajan (1979)). burned residual fuel droplets of around 3,000 µm initial diameter by using a H2-O2 micro torch at 2,200 °C. His observations included disruptive behaviour with large coke particles being expelled from the burning mass, after the burning of volatiles. These particles would leave an smoky trail behind.
It was also observed that combustion was not self-sustained, as when the heat source was removed the flame would be quenched. Only in the final stages the residue remaining after distillation and burning of the volatiles burned rather slowly.
Lightman and Street (Lightman and Street (1983)) and Marrone et al. (Marrone et al. (1984)) conducted studies of heavy oil droplet ignition that were published almost concurrently. Lightman and Street employed a heating lamp to irradiate droplets attached to silica fibres, and a gas flame to ignite the vapour ejected at heating rates similar to those experienced in real plant operation (approximately 104 °C/s). The samples studied included a range of residual oils of different geographical origin and varying carbon-forming propensity, their asphaltene and maltene fractions and, in a further division, the acetone-soluble and insoluble fractions of the maltenes.
Marrone et al. (Marrone et al. (1984)) suspended droplets of 1.0 to 1.5 mm on a quartz filament above a premixed C3H8-air flat-flame burner at an equivalence ratio of 0.8 and a temperature of 1,500 °C. An atmospheric residual oil, a high-sulphur no. 6 oil and a blend of residual oil and no. 2 oil were studied.
Lightman and Street divided the combustion processes into three separate stages: The pre-coking stage commenced with slight swelling and volatiles evolution under the form of granules around the droplet at 250 - 350 °C. It was followed by sharp ebullition at 300 - 400 °C and bubbling up to 800 °C along with a series of expansion and collapse alternating very rapidly with increasing diameter over twice the original size. Violent ejection of vapour also occurred, which was attributed by Marrone et al. (Marrone et al. (1984)) partly to volatiles produced by cracking.
It is in this stage that ignition begins, marked by radiation of high intensity.
The second stage (the coke formation) was marked by the end of volatiles evolution and the formation of a porous, rigid coke residue after final contraction, but before the end of the combustion of volatiles (Marrone et al. (1984)). The coke residues (studied under the Scanning Electron Microscope) were hollow and roughly spherical. The mass was concentrated in a shell of thickness only 5 % of the total diameter. The shell contained large holes and cavities. At the end of the coke formation the temperature had risen to 1,300 °C.
The third and final stage involves the burn-out of the coke residue; its diameter decreasing, pores widening and fragments being ejected. The complete disappearance of the particle marked the end of the burn-out stage. Peak temperatures of 1,400 - 1,500 were attained.
Malik and Burgess (Malik and Burgess (1985)) used silica-coated Pt/Pt 13%-Rh thermocouples of 25 µm diameter in a furnace and ciné camera recordings to obtain the behaviour history of droplets of as small sizes as 450 µm diameter. Various samples were analysed: Medium fuel oil, heavy fuel oil, atmospheric residue and gas oil.
Three clearly differentiated combustion stages were observed (see Figures 7 through to 10 (Malik and Burgess (1985))) :
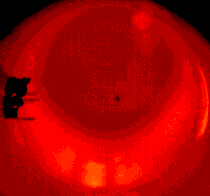 Figure 7: The pre-ignition delay period |  Figure 8: The flame period |
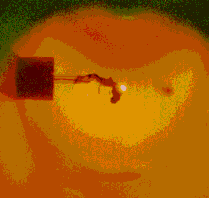 Figure 9: The coke-ignition delay period |  Figure 10: Soot residue left after combustion |
Malik and Burgess observed a linear relationship between the Pre-ignition Delay and the initial diameter of the droplet:
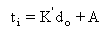
where:
ti: Pre-ignition Delay
K': slope
do: initial diameter
A: intercept.
Taylor and Burgess (Taylor and Burgess (1988)) studied a wide range of residual oils and atmospheric residues of varying geographic origin, and attempted to relate fuel composition to combustion parameters.
An inverse linear relationship was proposed between the Ignition Temperature and the initial diameter:
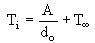
where T is a constant, possibly the ignition temperature of a large pool of oil (when do is very large). The inverse relationship of Ti with do proves that it is larger for small droplets than for large ones. The latter was explained as a consequence of the ease for larger droplets to produce enough vapour to reach the flammability limits of the fuel in the surroundings of the droplet, in comparison to small ones.
is a constant, possibly the ignition temperature of a large pool of oil (when do is very large). The inverse relationship of Ti with do proves that it is larger for small droplets than for large ones. The latter was explained as a consequence of the ease for larger droplets to produce enough vapour to reach the flammability limits of the fuel in the surroundings of the droplet, in comparison to small ones.
Other relationships of the combustion parameters with the initial droplet diameter for other combustion stages reported by Taylor and Burgess are:
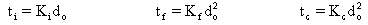
where:
ti: Pre-ignition Delay
tf: Flame Time.
tc: Coke Combustion Time
Ki, Kf, Kc: constants dependent upon the fuel type and the combustion conditions.
From their studies they concluded that Ki seemed to increase with high aromatic and low saturates content.
Like Masdin and Thring, Taylor and Burgess observed little variation of Kf in different fuels. They concluded that at high temperature, fuel composition is not relevant as long as the flame is established and it provides heat for evaporation and cracking.
Although Kc varied considerably throughout the group of fuels studied it showed a good, although not complete, relationship with the Conradson Carbon Residue:
which evidences a relationship between the coke combustion time and the amount of coke produced by the droplet.
 Previous |  Table of Contents |  Next |