2. Experimental procedure and results
The objective of the experiments reported in the present section is to estimate the maximum amounts of thermal-NOX emissions from the drop-tube furnace and also the conditions of equivalence ratio at which these are produced. An upper limit for thermal-NOX formation would thus be set and therefore lower amounts would be formed under any other conditions.
Experimental results reported in chapter III showed that maximum formation of NOX is attained at  = 0.833. Experiments reported in this section were carried out by setting three different stoichiometric ratios in the combustion chamber. The aforementioned equivalence ratio was investigated, as well as were those adjacent, namely fuel-lean (
= 0.833. Experiments reported in this section were carried out by setting three different stoichiometric ratios in the combustion chamber. The aforementioned equivalence ratio was investigated, as well as were those adjacent, namely fuel-lean ( = 0.714) and stoichiometric (
= 0.714) and stoichiometric ( = 1.000). This was accomplished by varying the amount of secondary air admitted in the combustion chamber whilst maintaining the same amount of atomisation air.
= 1.000). This was accomplished by varying the amount of secondary air admitted in the combustion chamber whilst maintaining the same amount of atomisation air.
The fuel used in this section was a commercially available diesel fuel . Typical physical and chemical characteristics of this fuel are reproduced in Table 9:
| Analysis
| Value
|
| Density @ 15 °C, kg/l
| 0.8563
|
| Kinematic viscosity @ 40 °C, cSt
| 2.0 - 4.5
|
| Sulphur, % by mass
| 0.05
|
| Nitrogen, ppm
| 230
|
| Water, mg/kg (max.)
| 200
|
| Carbon residue, % mass (max.)
| 0.30
|
| Particulate matter, mg/kg (max.)
| 24
|
| Gross calorific value, MJ/kg
| 45.72
|
Table 9: Typical chemical and physical characteristics of diesel fuel
Although the fuel used was not N-free, its low nitrogen content allowed the estimation of an upper limit for the formation of thermal-NOX.
The experiments were carried out at three furnace wall temperatures, namely 900 °C, 1,100 °C and 1,200 °C. Higher furnace wall temperatures would result in higher flame temperatures, which, in turn, would cause greater formation of thermal-NOX. As in experiments in chapter VII, sampling of combustion gases was performed at 500 mm from the atomiser nozzle.
The experimental results are presented and discussed in the next paragraphs.
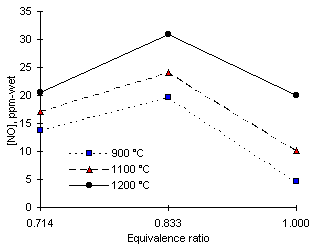
Figure 100: NO emissions from diesel fuel
The formation of NO from diesel fuel showed dependencies with both the flame temperature and the equivalence ratio (see Figure 100). Maximum amounts of NO were formed at approximately  = 0.833 at all furnace wall temperatures investigated. At 900 °C the maximum amount of NO emitted was 19.7 ppm-wet at
= 0.833 at all furnace wall temperatures investigated. At 900 °C the maximum amount of NO emitted was 19.7 ppm-wet at  = 0.833. Emissions of nitric oxide were lower at any other stoichiometric ratio. At
= 0.833. Emissions of nitric oxide were lower at any other stoichiometric ratio. At  = 0.714 the emissions of NO reached 13.8 ppm-wet, whereas at stoichiometric conditions only 4.6 ppm-wet were recorded.
= 0.714 the emissions of NO reached 13.8 ppm-wet, whereas at stoichiometric conditions only 4.6 ppm-wet were recorded.
As the furnace wall temperature was raised, the maximum formation of NO was also recorded at  = 0.833, although the values obtained were greater. The maximum concentration of NO measured was 30.9 ppm-wet at
= 0.833, although the values obtained were greater. The maximum concentration of NO measured was 30.9 ppm-wet at  = 0.833 and 1,200 °C furnace wall temperature.
= 0.833 and 1,200 °C furnace wall temperature.
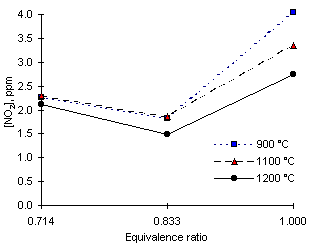
Figure 101: NO2 emissions from diesel fuel
The experimental results on NO2 emissions are shown in Figure 101. The values registered were low in all cases. At  = 0.714 the furnace wall temperature, and thus the flame temperature seemed not to have an influence on the amounts of NO2 emitted. However, under stoichiometric conditions the furnace wall temperature had an inverse effect on NO2. Lower amounts of NO2 were emitted as the furnace wall temperature was increased. Thus, the maximum values of NO2 emission, 4 ppm-wet, were recorded at
= 0.714 the furnace wall temperature, and thus the flame temperature seemed not to have an influence on the amounts of NO2 emitted. However, under stoichiometric conditions the furnace wall temperature had an inverse effect on NO2. Lower amounts of NO2 were emitted as the furnace wall temperature was increased. Thus, the maximum values of NO2 emission, 4 ppm-wet, were recorded at  = 1.000 and 900 °C furnace wall temperature.
= 1.000 and 900 °C furnace wall temperature.
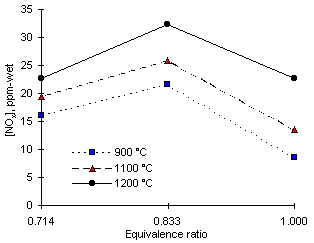
Figure 102: Total NOX (NO + NO2) from diesel fuel
Given the low amounts of NO2 formed at all equivalence ratios the behaviour of total NOX emissions followed the same pattern as those of NO. The maximum amounts of NOX formed at 900 °C furnace wall temperature and  = 0.833 were found to be 21.6 ppm-wet. At 1,200 °C and
= 0.833 were found to be 21.6 ppm-wet. At 1,200 °C and  = 0.833, 32.4 ppm-wet of total NOX were emitted.
= 0.833, 32.4 ppm-wet of total NOX were emitted.
Calculations of the conversion of fuel-N to NOX are provided in Figure 103, where the dotted horizontal line denotes 100 % conversion.
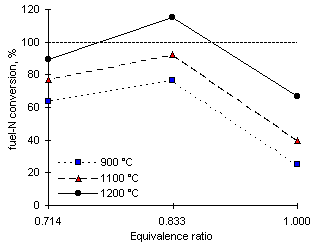
Figure 103: Conversion of fuel-N to NOX from diesel fuel
The conversion of fuel-N is relatively low at 900 °C furnace wall temperature. Again the maximum values are achieved at  = 0.833, with conversion close to 76 %, assuming that al NOX originate from nitrogen contained in the fuel. The conversion of fuel-N into NOX increases, as predicted, with increases of furnace wall temperature. The conversion reached a maximum value, 113.4 %, at
= 0.833, with conversion close to 76 %, assuming that al NOX originate from nitrogen contained in the fuel. The conversion of fuel-N into NOX increases, as predicted, with increases of furnace wall temperature. The conversion reached a maximum value, 113.4 %, at  = 0.833 and 1,200 °C. The fact that the conversion is higher than 100 % indicates that at least 3.8 ppm-wet thermal-NOX are formed in such conditions.
= 0.833 and 1,200 °C. The fact that the conversion is higher than 100 % indicates that at least 3.8 ppm-wet thermal-NOX are formed in such conditions.
From the results reported in the previous paragraphs it can be concluded that the emission of total-NOX from the diesel fuel is not greater than 22.6 ppm-wet at 900 °C furnace wall temperature and 0.833 equivalence ratio. Assuming that all nitrogenous emissions stem from the fuel-N (the fuel used contains 230 ppm fuel-N) is converted to NOX, and since fuel-NOX is formed at lower flame temperatures than thermal-NOX, it can be concluded that emissions of thermal-NOX are negligible at 900 °C furnace wall temperature.
A limited amount of thermal-NOX is formed at 1,200 °C furnace wall temperature and  = 0.833. Assuming that all fuel-N is converted to NOX, the emissions of thermal-NOX were 3.8 ppm-wet.
= 0.833. Assuming that all fuel-N is converted to NOX, the emissions of thermal-NOX were 3.8 ppm-wet.
Pollutant formation and interaction in the combustion of heavy liquid fuels
Luis Javier Molero de Blas, PhD thesis, University of London, 1998
© Luis Javier Molero de Blas
 = 0.833. Experiments reported in this section were carried out by setting three different stoichiometric ratios in the combustion chamber. The aforementioned equivalence ratio was investigated, as well as were those adjacent, namely fuel-lean (
= 0.833. Experiments reported in this section were carried out by setting three different stoichiometric ratios in the combustion chamber. The aforementioned equivalence ratio was investigated, as well as were those adjacent, namely fuel-lean ( = 0.714) and stoichiometric (
= 0.714) and stoichiometric ( = 1.000). This was accomplished by varying the amount of secondary air admitted in the combustion chamber whilst maintaining the same amount of atomisation air.
= 1.000). This was accomplished by varying the amount of secondary air admitted in the combustion chamber whilst maintaining the same amount of atomisation air.






