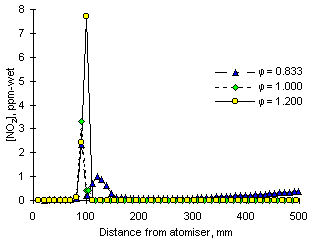
Figure 116: Calculated concentrations of NO2 from fuel M1 at three equivalence ratios

In the low temperatures of the CSTR zone the formation of NO2 is low, due to the similarly reduced concentrations of NO and the absence on HO2 radicals, which give rise to NO2 formation mainly through reaction with NO.
As the flame temperature increases so does the concentration of NO2, with higher values as oxygen is consumed and the combustion conditions become fuel-rich. This process continues until the end of the CSTR zone. On entering the PFR zone the concentrations of NO2 drop very rapidly, and its emissions become negligible at all equivalence ratios.
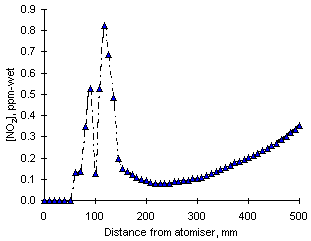
 = 0.833
= 0.833The results of NO2 emissions calculations from Orimulsion are shown in Figure 117. The calculated values of NO2 emissions were, again, much lower than those obtained experimentally. The PFR regime causes a decrease of the concentrations of NO2, which leads to very low emissions. Emissions of nitrogen dioxide obtained experimentally were 33.5 ppm-wet.
Why are NO2 emissions so different to experimentally obtained values?
The formation of HO2 radicals according to the numerical simulation is high in the CSTR zone, particularly in fuel-rich conditions. However, virtually all HO2 radicals are consumed rapidly in the PFR zone. Calculations of the contributions of individual reactions showed that reaction with H radicals:
 OH + OH reac 97
OH + OH reac 97is responsible for the abrupt disappearance of HO2 radicals, which are then no longer able to contribute to the formation of nitrogen dioxide, as can be seen in Figure 118.
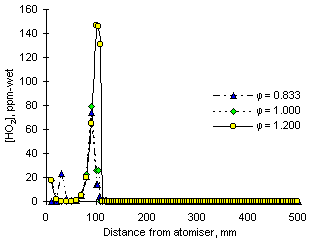
Table 12 shows the reactions containing NO2 comprised in the numerical model, whereas Figure 119 presents a graph of the evolution of species involved in the disappearance of NO2 from fuel M1 in fuel-rich conditions:
NO2 is consumed in stoichiometric and fuel-rich conditions predominantly by means of H and O radicals in reactions:
NO2 + O  NO + O2 NO + O2
| reac 30 |
NO2 + H  NO + OH NO + OH
| reac33 |
The overall effect of these reactions with the high concentrations of H and O radicals shown in Figure 119 is a sharp drop of NO2 concentrations in the PFR zone, which causes low values of NO2 at exhaust.
Profiles of nitrogen dioxide similar to those obtained in this numerical model were experimentally obtained by Merryman and Levy (Merryman and Levy (1974)) in stoichiometric and fuel-rich O2/CH4 flames. Relatively high concentrations of NO2 were observed in the early stages of the flame, rapidly decaying subsequently. These researchers attributed the formation of NO2 exclusively to reactions between NO and HO2 radicals.
Several other researchers (Bromly et al. (1988), Hori (1988), Jaasma and Borman (1980)) have suggested in the past that large amounts of NO2 can be formed in fuel-rich mixtures of hydrocarbon fuels. This is due to the presence of unburned hydrocarbons which may enhance HO2 formation and may also supply hydroperoxyl radicals (RO2). Experimental results obtained in this thesis (Burgess and Molero (1996)) show that large amounts of NO2 are emitted at stoichiometric and, especially, fuel-rich conditions (see chapter III). Thus, a likely source of error in the estimation of NO2 concentrations in stoichiometric and fuel-rich conditions may be the reactions of NO with hydrocarbon fractions (hydroperoxyl radicals, RO2). These reactions were absent from the reaction scheme considered in this thesis, and they may also account for the erroneous concentrations of NO calculated at  = 1.000 and 1.200.
= 1.000 and 1.200.
Therefore, an extended set of species and reactions is necessary in order to accurately simulate the formation of NO2 in the drop-tube furnace at stoichiometric and fuel-rich equivalence ratios.
 Previous |  Table of Contents |  Next |