
Two consecutive processes can be distinguished in the emission of coke particulates (Urban and Dryer (1990), Urban and Dryer (1990), Urban and Dryer (1990)) , namely particle formation followed by its oxidation or burnout. The process of particle formation is strongly dependent upon the fuel properties, although mostly independent of the combustion environment (Urban and Dryer (1990)) . On the contrary, the particle oxidation process is affected by the combustion environment, but also by the fuel composition as it provides the material for the coke particle.
The stages of the combustion of heavy fuel oil droplets are described in section "2.1. Combustion of heavy fuels and emulsions". Droplets undergo considerable swelling before and during ignition, although a large part of the mass is expelled by fractional distillation and pyrolysis. Coking starts when a threshold temperature is reached, lower than that of soot formation but higher than that of distillation. It lasts only for a few milliseconds, in the last 10 to 15 % of the droplet vaporisation time (Marrone et al. (1984)) . An immature tarry coke particle appears 15 ms prior to the flame quenching. This immature particle eventually becomes a coke particle of much larger size than the original droplet but of reduced mass.
The structural characteristics of coke particles reveal that the non-volatile components that remain after combustion of the volatile fractions provide the material for the cenospheres. Their mass is related to the asphaltene content of the fuel, but also to the amount of maltene and resin components (Burgess (1990), Marrone et al. (1984)) . Fuels containing aromatics form coke more readily than others (Glassman (1986)) . However, the mass of the coke particle is independent of the diameter of the initial droplet (Marrone et al. (1984)) .
Scanning Electron Microscopy is a useful tool for the characterisation of cenospheres. Clayton and Back (Clayton and Black (1989)) showed cenospheres to be hollow and spherical, and with at least one large "blowhole" through the shell. Their structure shows porous layers (the volume of the solid material represents only 18 % of the total envelope volume), which provide effective porosity for oxidation reactions (Clayton and Black (1989)) . Urban and Dryer (Urban and Dryer (1990)) reported differences in pore abundance between the inner side and outer surface. Also, pores seem to concentrate on certain regions of the surface, ie, it is not a uniform process.
Detailed examination by the EDS technique (Clayton and Black (1989), Urban and Dryer (1990)) provides an insight of the chemical composition of cenospheres, which varies with size: In particles larger than 10 µm diameter the C content exceeds 80 %, whereas for smaller particles it is reduced to 70 % (Dryer and Kerho (1987), Goldstein and Siegmund (1976)) . Cenospheres analysed by Clayton and Back (Clayton and Black (1989)) were composed mainly of C, N, H and P. High concentrations of S, Na, Mg, Fe, V and Ca were also found. According to Urban and Dryer (Urban and Dryer (1990)) , catalytic elements such as V and Si are evenly distributed on the particle surface, therefore they are not responsible for large blowholes. However, Si is present only on the outer surface, indicating that it may condense onto the surface as the particle is quenched.
The role of sulphur in coke burn-out has not been clarified yet as contradictory effects have been described (Burgess (1990)) . Sulphur in heavy fuels is normally associated with the heavy fractions of the fuel. It may either diminish the catalytic activity of metals or increase the oxidation rate of the carbonaceous particle. X-ray and diffraction microscopy show (Dryer and Kerho (1987)) that fuel-S is uniformly distributed in the particulates, and is released only in the latter stages of combustion. Goldstein and Seigmund (Goldstein and Siegmund (1976)) studied the effect of blending a high-S fuel and a S-free fuel to simulate a low-S fuel. The effect of dilution was to diminish particulate emissions: When S was reduced from 2.2 % to 0.5 % the total particulate emission decreased by 75 %.
The burn-out of cenospheres (Kelly et al. (1989)) is much slower than the liquid phase vaporisation processes in droplets. Oxidation is controlled by diffusion in pores, and its rate depends upon the chemical reactivity of the coke, temperature, oxygen concentration, size and porosity of the particles. However, some authors argue that given the normal pore size, diffusion is not a limiting factor for oxidation, and therefore it also takes place on the internal surface (Urban and Dryer (1990)) . Also the catalytic action of metals contained (V and Ca) (Taylor and Burgess (1988)) may accelerate burn-out, although paradoxically, their contribution seems to be rather unnecessary at such high temperatures (around 1,800 °C).
Exhaustive work on particulates from power station boilers carried out by Cunningham and Datschefski (Cunningham and Datschefski (1981)) led to the conclusion that the asphaltene and vanadium contents are the most important variables related to particulate emissions, which was also confirmed experimentally by Taylor and Burgess (Taylor and Burgess (1988)) . However, they obtained scattered results when attempting a relationship between particulate emissions at 4 % oxygen and the Conradson Carbon Residue, as an indicator of the carbon forming tendency of a fuel.
Whitehead et al. (Whitehead et al. (1983)) and later Kelly et al. (Kelly et al. (1989)) found best correlation of the particulate formation in atmospheric residues with the following equation which shows the complex nature of the coke formation and burnout process:

in which:
PC: particulate carbon emissions (no ash) at 4 % excess O2, % by weight in fuel
Plant factor: a proportionality constant particular for each set of operating conditions
Asphaltenes: asphaltene content of the fuel
TLC resins: residue measured by Thin Layer Chromatography
S: sulphur content in the fuel, % by weight
V, Na: vanadium and sodium contents, ppm.
King (King et al. (1981)) reports the following formula which, although specific for his study, takes both particle formation and burnout processes into account:
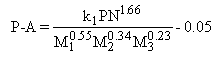
where:
P: particulates, % mass/mass fuel
A: fuel ash content, % mass/mass fuel
k1: constant
PN: fuel pentane insolubles
M1: (V + Ni + Fe + Mn) in fuel, mg/kg
M2: Ca in fuel, mg/kg
M3: Na in fuel, mg/kg
Later Urban and Dryer (Urban and Dryer (1990)) proposed a parameter called the Coke Formation Index (CFI) as the ratio of the coke particle mass to the initial droplet mass before the onset of burn-out in free, isolated droplet combustion experiments. The Coke Formation Index (Urban and Dryer (1990)) was based on the geometric and physical characteristics of both fuel and coke particle only:
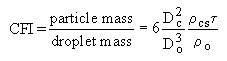
where:
Dc: coke particle diameter
Do: droplet diameter
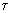 : coke particle shell thickness ratio
: coke particle shell thickness ratio
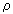 o: density of fuel droplet
o: density of fuel droplet
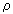 cs: density of the coke particle shell
cs: density of the coke particle shell
The Coke Formation Index is mostly invariant over a wide range of droplet sizes, temperature profiles and oxygen concentrations (Urban and Dryer (1990)) , but strongly influenced by the fuel type. Its values are maximum at the point where the vapour-phase flame extinguishes. Subsequently it decreases as both oxidation and burnout progress.
A relationship between CFI and the fuel composition was also pursued by Urban and Dryer (Urban and Dryer (1990)) . The CFI seems to correlate with the heptane asphaltene content (ASP), according to:
Other metals tested by Feldman (Feldman (1982)) can be ordered according to their ability to reduce particulate formation:
where Mn and Mg do not cause reduction and Ba caused increase of particulates due to the formation of stable vanadates, thus reducing V availability.
Fe is reported to form high melting point, non-toxic, non-fouling and non-corrosive deposits. Cunningham (Cunningham (1978)) reports that addition of 130 ppm of organically-bound Fe reduced particulates by 60 %.
 Previous |  Table of Contents |  Next |