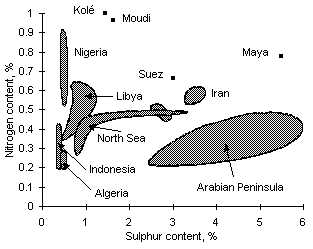
Figure 1: Sulphur and nitrogen contents in vacuum residues according to their geographical origin
Heavy oils are products derived from crude oils. Thus, their composition varies with that of the source crude. They are composed entirely, or substantially, of the residuals or bottoms from petroleum refining operations, ie, materials which remain in a condensed form in the processing. The atmospheric distillation temperature for these components exceeds 540 °C, and they appear after all other lighter products have been removed from the refinery stream.
Some physical and chemical properties of heavy oils are given in the following Table as a function of the sulphur content:
| Property | High sulphur | Intermediate sulphur | Low sulphur |
|---|---|---|---|
| Sulphur, % by weight | 2.2 | 0.96 | 0.50 |
| Carbon, % by weight | 86.25 | 87.11 | 87.94 |
| Hydrogen, % by weight | 11.03 | 10.23 | 11.85 |
| Nitrogen, % by weight | 0.41 | 0.26 | 0.16 |
| API gravity | 17.3 | 21.5 | 24.7 |
| Viscosity, cSt @ 38 °C | 690 | 129 | 49 |
| Conradson Carbon, % by weight | 12.51 | 5.64 | 2.43 |
| Hexane insoluble, % | 10.33 | 4.72 | 2.25 |
| Ash, % | 0.08 | 0.04 | 0.02 |
| Metals, ppm: | |||
| Vanadium | 350 | 155 | 70 |
| Nickel | 41 | 20 | 10 |
| Sodium | 25 | 10 | <5 |
| Iron | 13 | 9 | <5 |

Since heavy oils are some of the last products from crude refining, and their production is subject to that of other more profitable streams, the quality and characteristics of those materials fluctuate from one refinery to another. Until the mid seventies oil crisis, variations in heavy oil composition were small due to the continuity of suppliers and their uniform refining techniques. This was helped also by the less strict environmental regulations in force by then. As the crisis deepened in the late seventies and environmental regulations became more rigid, a shift in oil sources lead to an increase of sulphur content and increases of other contaminants of reduced volatility.
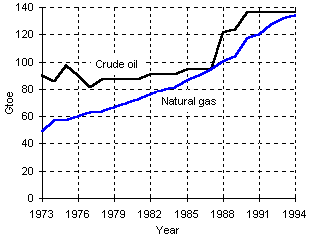
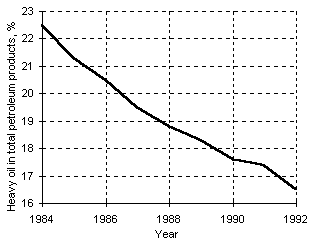
Whilst the World reserves of crude oil and natural gas increased during the last twenty five years (see Figure 2), the production and consumption of heavy oils decreased (Figure 3), giving way to lighter, more valuable compounds and residual products with lower H content and heating value (Raimbault (1995)) .
Refineries have been adapted to intensify the production of light compounds in processes like that shown in Figure 4. For instance, visbreaking units (intermediate temperature, high pressure cracking) are being added to existing processes. Visbreaking produces additional volatile materials and a "cracked" residual material. The volatiles so produced may be used either to increase the yield of light products or to replace gas oil as a "cutter" stock for adjusting the viscosity characteristics of heavy oil by blending with the residual fractions.
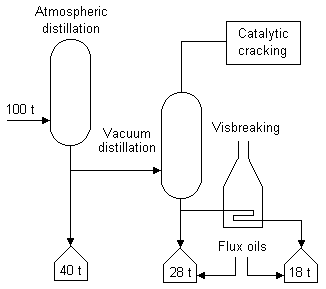
The "cracked" material produced by visbreaking has higher asphaltene content with different characteristics than that in the original distillation residua. Since N, S and ash components tend to concentrate in the residua, their amounts are directly related to the residual fraction in the final blend.
Delayed coking is another process which increases the proportion of light products, with total transformation of residues into gases, condensates, gasolines, distillates and coke. Delayed coking is performed at 500 °C and 5 bars at a very low rate. The products are subsequently separated by distillation, although the large formation of coke in the chambers makes it necessary to run the process in cycles so that these deposits can be removed.
An additional practice in heavy fuel oil commercialisation is the marketing by "jobbers", agents who blend heavy oils to meet specifications, thus making it difficult to track the original source and quality of the oil. Heavy oils can be supplied as blends of residual and distillate materials, ie, "reduced" crude (crude which has had only the most volatile fractions removed) or "straight run" residues (fluid residue after atmospheric and/or vacuum distillation). Blending may give rise to incompatibility problems with precipitation of non-compatible components which may cause clogging and other handling difficulties.
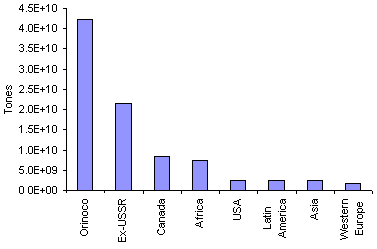
Heavy oils are also a natural occurrence in some parts of the World. They represent a substantial part of the petroleum reserves, almost as large as those of crude oil. A comparative chart of heavy hydrocarbon reserves is presented in Figure 5. In particular, the Orinoco belt contains the World's largest reserves of heavy crude oil and bitumen.
Emulsification is the most viable way for heavy crude oil exploitation. An emulsion can be defined as a heterogeneous system of liquid phases, consisting of at least one immiscible liquid dispersed in another as droplets. The liquid that is broken into droplets is the internal or dispersed phase, whereas the liquid surrounding the droplets is called the continuous or external phase.
The commercial name of oil:water emulsions from the Orinoco area is Orimulsion. The industrial process of Orimulsion production starts with the extraction of the crude oil. This is carried out by pumping water underground, forming a primary emulsion that contains some amounts of gas. The gas is subsequently stripped in heat exchangers. The primary emulsion is then broken, and the pure oil goes to a reformation process. The final oil:water emulsion is formed by mixing with 30 % desalinated water containing surfactants of the type onylphenol ethoxylate. In some cases residual oils contain sufficient amounts of natural surfactants that stabilise the emulsion for long periods of time.
The large vanadium and sulphur of Orimulsion presents difficulties for refining, basically due to interaction with catalysts. However, its low viscosity facilitates handling, pumping and transportation. Orimulsion can also be burnt in boilers and heaters, and this is one of its most successful applications.
 Previous |  Table of Contents |  Next |